Evaluation of vascular invasion in hepatic alveolar echinococcosis using computed tomography: An observational study
ORIGINAL RESEARCH ARTICLE
Evaluation of vascular invasion in hepatic alveolar echinococcosis using computed tomography: An observational study
Article Summary
- DOI: 10.24969/hvt.2024.553
- INTERNAL MEDICINE
- Published: 15/03/2025
- Received: 19/11/2024
- Revised: 27/01/2025
- Accepted: 27/01/2025
- Views: 4223
- Downloads: 2200
- Keywords: Alveococcosis, alveolar echinococcosis, echinococcus multilocularis, computed tomography, liver, liver vessels
Address for Correspondence: Iliar A. Baudinov, Aliya I. Kadyrova, Radiology Department, I.K. Akhunbaev Kyrgyz State Medical Academy, 92A, Akhunbaev str, 720020, Bishkek, Kyrgyz Republic
Phone: Iliar A. Baudinov - +996555772660, Aliya I. Kadyrova - +996555934406
Email: Iliar A. Baudinov - ili4bia@gmail.com, Aliya I. Kadyrova - al-kadyrova@yandex.ru
Ilyar A. Baudinov1a*, Aliya I. Kadyrova1a*, Kanat Omorov1b, Bakhadyr K. Bebezov1c, Kubat Ibraimov1a, Aleksei Oginskiy1a, Roshan Kumar Shah1a, Shabdan N. Isamadyrov1a
1aDepartment of Radiology, 1bDepartment of Faculty Surgery named after Academician K.R. Ryskulova and 1cDepartment of Innovative Surgical Technologies, I.K. Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
Abstract
Objective: Alveolar echinococcosis (AE) is a severe, chronic parasitic disease predominantly affecting the liver. Surgical intervention is the only definitive treatment, but vascular invasion significantly increases surgical risks. This study aimed to identify factors associated with vascular invasion in hepatic AE to improve diagnosis and surgical planning.
Methods: A retrospective analysis of 70 patients with newly diagnosed hepatic AE was conducted using contrast enhanced computed tomography (CT). Factors including sex, age, number and size of foci, lesion volume, and the morphologic type of lesions (based on the Alveolar Echinococcosis Ulm Classification, AEUC) were evaluated for their association with blood vessel invasion. Statistical analyses included cross-tabulations and symmetric measures, with significance assessed at p < 0.05.
Results: Among the variables analyzed, the number of foci and the size of the largest focus were identified as significant predictors of vascular invasion, with Cramer's V values of 0.404 (p=0.022) and 0.630 (p=0.066), respectively. Other factors, including sex, age, lesion volume, and AEUC morphological type, showed weaker or statistically insignificant associations with vascular involvement.
Conclusion: The invasion of parasitic nodules into large blood vessels in patients with first diagnosed hepatic AE does not depend directly on sex, age, type of focus according to AEUC. It can be stated that with a high probability invasion of pathologic focus into the vascular wall is associated with an increase in the volume of liver lesion. The most significant predictors of invasion into large vessels are the number of foci and the size of the largest foci.
Graphical abstract
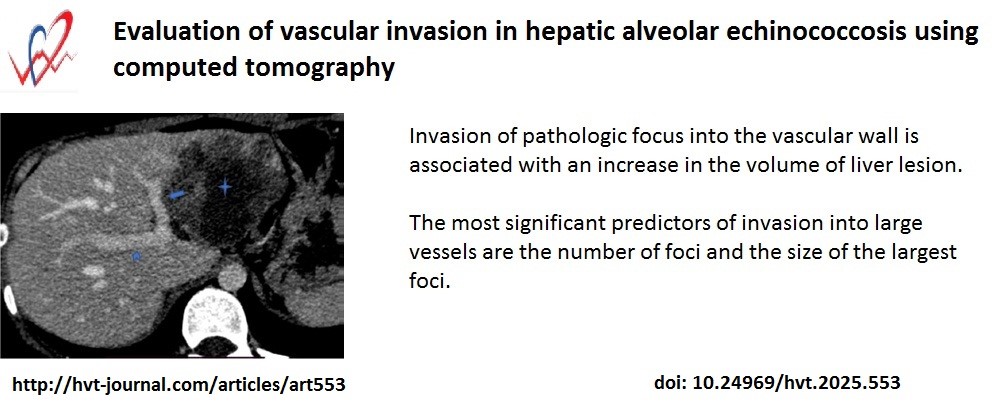
Key words: Alveococcosis, alveolar echinococcosis, echinococcus multilocularis, computed tomography, liver, liver vessels
Introduction
Alveolar echinococcosis (AE) of the liver is a chronic and severe parasitic disease caused by the larval stage of Echinococcus multilocularis (1). AE is predominantly found in the northern hemisphere, with natural reservoirs located in Central Asia (e.g., Kazakhstan, Kyrgyzstan, northern Pakistan, and western China), Europe (e.g., Poland, Germany, Austria, Switzerland), Asia (e.g., China, Japan), and North America (e.g., Alaska) (2). In the Kyrgyz Republic, some regions report postoperative morbidity rates as high as 246 per 100,000 population (3). In most cases, the liver is the primary organ affected by AE (4). The spread of parasitic masses typically follows the veins of the portal system and the intrinsic venous system of the liver, often involving the inferior vena cava (5).
Contrast enhanced computed tomography (CECT) is a cornerstone imaging modality in diagnosing hepatic alveolar echinococcosis. Its ability to provide detailed visualization of lesions, evaluate vascular and biliary involvement, and guide surgical planning makes it indispensable. By enabling accurate diagnosis, staging, and treatment monitoring, CECT significantly improves patient outcomes in this complex parasitic disease. Surgical intervention remains the only definitive treatment for hepatic alveolar echinococcosis (6). Invasion of the parasitic tumor into the vascular wall can greatly complicate surgical procedures and increase the risk of complications (7). Identifying factors that influence the contact between parasitic nodules and vessel walls is crucial for diagnosis, surgical planning, and outcome prediction.
This study evaluated factors such as sex, age, lesion volume, and the number, size, and morphology of foci based on the AEUC (Alveolar Echinococcosis Ulm Classification), which includes five types (8), to determine their association with contact with major blood vessels. Special emphasis was placed on assessing whether the morphological characteristics of the parasitic nodule impact the likelihood of vascular invasion.
Methods
Study design and population
This was a retrospective observational study. CT data from 70 patients diagnosed with hepatic AE were analyzed. The population age group included ranges from 10 to 77 years. Patients referred to medical diagnostic center “Sistem” between January 2019 and December 2023 were analyzed.
Ethical approval was obtained from ethical committee of I. K. Akhunbaev Kyrgyz State Medical Academy. Informed consent was obtained from all patients for diagnostic procedures.
Baseline variables
The following variables were included in the analysis: sex, age at the time of the study.
Imaging and criteria for vascular invasion
Diagnosis of hepatic AE was made by using CECT. The CT- scan machine used was GE healthcare Light speed VCT and triple phase contrast study was done by Iodixanol 320 100-120 mL (3 to 5 mL/s).
We evaluated the contact between parasitic nodules and major liver vessels, including the portal vein trunk, its right and left branches, the common hepatic artery, the proper hepatic artery, the right and left branches of the hepatic artery, the right, median and left hepatic veins at their confluence with the inferior vena cava and the inferior vena cava itself. Imaging criteria for vascular invasion included:
1. Loss of fat plane: The absence of the fat plane separating the nodule from the vessel was considered a sign of close contact.
2. Deformity or displacement of the vessel: Any visible change in the vessel’s course or contour due to compression or displacement by the parasitic nodule.
3. Encasement of the vessel: parasitic nodule covering more than 180 degree of the vessel’s circumference.
4. Contact with vessel: contact between the vessel and the parasitic node measuring 20mm or more.
5. Wall Irregularity or Indentation of the vessel: an irregular or indented appearance of the vessel wall adjacent to the nodule was taken as evidence of nodule-vessel contact.
6. Thrombosis or stenosis: partial or complete thrombosis or narrowing of the vessel lumen was noted if present.
7. If at least one of the specified criteria of contact with any of the listed vessels was present, the case was classified as positive for this feature (Fig. 1, Fig. 2).
Computed tomography variables
The number of foci (1 to 5 or more), the maximum size of the largest foci in centimeters, the total volume of the affected liver sectors, and the type of the largest foci according to the AEUC classification were also taken into account. The AEUC classification of alveolar echinococcosis according to computed tomography is divided into 5 types (8).
Type 1 - small cystic/metastasis-like, characterized by non-flat, homogeneous nodules with minimal signs of degeneration, without calcification or with central calcification.
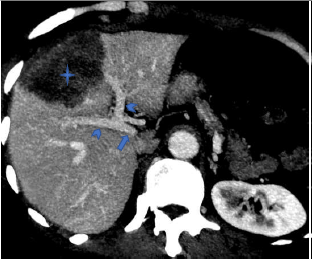
Figure 1. Computed tomography of abdomen (venous phase): The portal vein (arrow) and its main branches (arrow heads) have no contact with the parasitic mass (star)
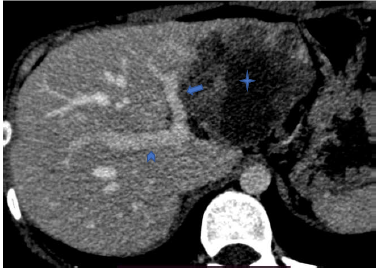
Figure 2. Computed tomography of abdomen (venous phase): The right branch of portal vein (arrowhead) has no contact with the parasitic mass (star). The left branch of the portal vein (arrow) is adjacent to the mass, with contact measuring more than 20 mm and an uneven internal contour.
Type 2 - diffuse-infiltrative, with indistinct irregular contours, irregular oval or branching shape, may contain cystic inclusions (fluid necrosis), delicate small focal and linear calcification.
Type 3 - predominantly circumscribed, tumor-like, has fairly distinct contours, convex shape, may contain large cystic areas and focal calcification.
Type 4 - predominantly cystic, has fairly clear contours, medium or large size, oval or round shape with a dominant cystic component (necrosis), pronounced diffuse calcification.
Type 5 - predominantly calcified, the dominant part of the focus is represented by calcified tissue with small islets of necrotic tissue.
Statistical Analysis
SPSS version 23 (IBM Corp., New York, USA) was used for statistical analysis. Associations between variables and vascular invasion were evaluated using Cramer’s V and p-values. Statistical significance was set at p < 0.05.
Results
The results of the study are presented in the respective tables.
Sex: Out of 70 patients, 39 were females and 31 were males (Table 1). Positive contract of nodes with vessels was observed in 33 women and 30 men.
|
Table 1. Sex distribution and vascular Invasion |
|||||
|
Cross table |
|||||
|
Count |
|||||
|
Variable |
Sex |
Total |
|||
|
female |
male |
||||
|
Invasion of large blood vessels |
No invasion |
6 |
1 |
7 |
|
|
With invasion |
33 |
30 |
63 |
||
|
Total |
39 |
31 |
70 |
||
|
Symmetric measures |
|||||
|
|
Significance |
Approximate significance |
|||
|
Nominal/nominal |
Phi |
0.201 |
0.092 |
||
|
Cramer's V |
0.201 |
0.092 |
|||
|
Number of admissible observations |
70 |
|
|
||
Statistical analysis showed no significant correlation between gender and large vascular invasion (Cramer's V = 0.201, p = 0.092) indicating that there was no statistically significant association between sex and large vessel invasion.
Age: The mean age was 42.8 (12.7) years. The age of patients without invasion ranged from 20 to 44 years, with individual cases older than 48 years. Among patients with invasion, age was wider, ranging from 10 to 77 years, indicating a more even distribution across age groups.
As can be seen from Table 2, the Phi coefficient (0.768) and Cramer's V (0.768) indicate a strong association between vascular invasion and patient age, but the p value = 0.288 indicates that this relationship is not statistically significant.
|
Table 2. Relationship between age and vascular invasion |
|
|||||||||||||||||||||||||||||||||||
|
Cross - table |
|
|||||||||||||||||||||||||||||||||||
|
Count |
|
|||||||||||||||||||||||||||||||||||
|
Variable |
Age at the time of the study (full years) |
|
||||||||||||||||||||||||||||||||||
|
10 |
12 |
13 |
15 |
19 |
20 |
23 |
24 |
25 |
26 |
|
||||||||||||||||||||||||||
|
Invasion of large blood vessels |
No invasion |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
|
||||||||||||||||||||||||
|
With invasion |
1 |
1 |
2 |
1 |
1 |
0 |
1 |
2 |
4 |
2 |
|
|||||||||||||||||||||||||
|
Total |
1 |
1 |
2 |
1 |
1 |
1 |
1 |
2 |
4 |
2 |
|
|||||||||||||||||||||||||
|
Variable |
Age at the time of the study (full years) |
|
||||||||||||||||||||||||||||||||||
|
27 |
28 |
29 |
30 |
31 |
33 |
35 |
36 |
37 |
38 |
|
||||||||||||||||||||||||||
|
Invasion of large blood vessels |
No invasion |
1 |
0 |
1 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
|
||||||||||||||||||||||||
|
With invasion |
2 |
4 |
0 |
0 |
3 |
3 |
1 |
2 |
4 |
1 |
|
|||||||||||||||||||||||||
|
Total |
3 |
4 |
1 |
1 |
4 |
3 |
1 |
2 |
4 |
1 |
|
|||||||||||||||||||||||||
|
Variable |
Age at the time of the study (full years) |
|
||||||||||||||||||||||||||||||||||
|
40 |
41 |
42 |
43 |
44 |
45 |
48 |
51 |
52 |
53 |
|
||||||||||||||||||||||||||
|
Invasion of large blood vessels |
No invasion |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
|
||||||||||||||||||||||||
|
With invasion |
4 |
1 |
2 |
1 |
2 |
2 |
1 |
1 |
1 |
1 |
|
|||||||||||||||||||||||||
|
Total |
4 |
1 |
2 |
1 |
3 |
2 |
1 |
2 |
1 |
1 |
|
|||||||||||||||||||||||||
|
Variable |
Age at the time of the study (full years) |
Total |
|
|
||||||||||||||||||||||||||||||||
|
56 |
57 |
60 |
61 |
62 |
63 |
69 |
77 |
|
||||||||||||||||||||||||||||
|
Invasion of large blood vessels |
No invasion |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
7 |
|
|||||||||||||||||||||||||
|
With invasion |
3 |
3 |
1 |
1 |
1 |
1 |
1 |
1 |
63 |
|
||||||||||||||||||||||||||
|
Total |
3 |
3 |
1 |
1 |
1 |
1 |
1 |
1 |
70 |
|
||||||||||||||||||||||||||
|
Symmetric measures |
|
|||||||||||||||||||||||||||||||||||
|
|
Significance |
Approximate significance |
|
|||||||||||||||||||||||||||||||||
|
Nominal/nominal |
Phi |
0.768 |
0.288 |
|
||||||||||||||||||||||||||||||||
|
Cramer's V |
0.768 |
0.288 |
|
|||||||||||||||||||||||||||||||||
|
Number of admissible observations |
70 |
|
|
|
||||||||||||||||||||||||||||||||
Number of foci in the liver: The distribution of patients according to the number of foci in the liver shows that among those without large vessel invasion, the majority had only one foci (4 of 7 patients), and only two patients had 2 or 3 foci. Whereas among patients with vascular invasion, the main number (44 out of 63) also had one foci, but a larger number of patients with two or more foci were represented. Cramer's V's is 0.404, indicating that there was a moderate association between vascular involvement and the number of foci in the liver.
As can be seen from Table 3, the approximate significance (p=0.022) is below the threshold value (p<0.05), indicating that the association is statistically significant. Hence, it can be concluded that there is a moderate but statistically significant relationship between these variables.
|
Table 3. Impact of number of liver foci on vascular invasion |
||||||||
|
Cross table |
||||||||
|
Count |
||||||||
|
Variable |
Number of foci in the liver |
Total |
||||||
|
1 |
2 |
3 |
4 |
5 or more |
||||
|
Invasion of large blood vessels |
No invasion |
4 |
1 |
2 |
0 |
0 |
7 |
|
|
With invasion |
44 |
15 |
1 |
1 |
2 |
63 |
||
|
Total |
48 |
16 |
3 |
1 |
2 |
70 |
||
|
Symmetric measures |
|
|||||||
|
|
Significance |
Approximate significance
|
||||||
|
Nominal/nominal |
Phi |
0.404 |
0.022
0.022
|
|||||
|
Cramer's V |
0.404 |
|||||||
|
Number of admissible observations |
70 |
|||||||
CT morphology of foci, AEUC: Of the 70 patients presented in the study, among those who did not have an invasion into large vessels, patients with foci of types 3 and 4 according to AEUC were observed (5 and 2 patients, respectively) (Table 4). In contrast, type 3 (30 patients) and type 4 foci (18 patients) predominated among patients with large vessel invasion. At the same time, there were also patients with foci of types 1, 2, and 5, which confirms the diversity of morphological characteristics of foci in the group with invasion.
|
Table 4. Association between morphological type of foci (AEUC) and vascular invasion |
|||||||||
|
Cross table |
|||||||||
|
Count |
|||||||||
|
Variable |
Type of largest focus by AEUC |
Total |
|||||||
|
1 type |
Type 2 |
3 type |
Type 4 |
5 type |
|||||
|
Invasion of large blood vessels |
No invasion |
0 |
0 |
5 |
2 |
0 |
7 |
||
|
With invasion |
2 |
8 |
30 |
18 |
5 |
63 |
|||
|
Total |
2 |
8 |
35 |
20 |
5 |
70 |
|||
|
Symmetric measures |
|
||||||||
|
|
Significance |
Approximate significance |
|||||||
|
Nominal/nominal |
Phi |
0.184 |
0.666 |
||||||
|
|
Cramer's V |
0.184 |
0.666 |
||||||
|
Number of admissible observations |
70 |
|
|||||||
Heart, Vessels and Transplantation 2025; 9: doi: 10.24969/hvt.2025.553
CT evaluation of vascular invasion in hepatic alveococcosis Baudinov et al.
![]()
Table 4 shows Phi and Cramer's V coefficients of 0.184 that indicate a weak association between the type of nidus and invasion of large blood vessels, while a p value of 0.666 indicates that this association is not statistically significant. Therefore, based on the data, it can be concluded that the type of the largest focus according to the AEUC classification has no significant effect on the probability of invasion into large blood vessels.
Size of the largest nidus: The study analyzed the relationship between the size of the largest nidus and invasion of large blood vessels in a sample of 70 patients. The Table 5 shows that among patients without invasion (7 patients), the most frequent foci were 5.0 cm and 6.0 cm in size. Whereas, in the group of patients with invasion into large vessels (63 patients), foci sizes ranged from 5.0 mm to 24.0 cm, with the largest proportion of patients having foci between 10.0 cm and 16.0 cm, indicating more common large foci in this group.
Cramer's V of 0.630 indicates a strong association between foci size and vascular invasion. However, a p value of 0.066 indicates that this relationship is not statistically significant at the 0.05 level, although it is close to significance. This suggests that although there is a trend toward increased foci size in patients with invasion, the data do not confirm this with a high degree of certainty. Thus, it is conceivable that larger foci size is associated with an increased risk of invasion into large vessels, but this statement requires more evidence.
|
Table 5. Correlation between largest nidus size and vascular invasion |
||||||||||||||||
|
Cross table |
||||||||||||||||
|
Count |
|
|||||||||||||||
|
Variables |
Size of the largest nidus |
|||||||||||||||
|
5 |
6 |
7 |
8 |
9 |
10 |
11 |
||||||||||
|
invasion of large blood vessels |
No invasion |
1 |
2 |
1 |
1 |
1 |
0 |
0 |
||||||||
|
With invasion |
3 |
0 |
2 |
5 |
3 |
6 |
4 |
|||||||||
|
Total |
4 |
2 |
3 |
6 |
4 |
6 |
4 |
|||||||||
|
Variables |
Size of the largest nidus |
|||||||||||||||
|
12 |
13 |
14 |
15 |
16 |
17 |
18 |
||||||||||
|
invasion of large blood vessels |
No invasion |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
||||||||
|
With invasion |
4 |
5 |
2 |
7 |
9 |
2 |
3 |
|||||||||
|
Total |
5 |
5 |
2 |
7 |
9 |
2 |
3 |
|||||||||
|
Variables |
Size of the largest nidus |
Total |
||||||||||||||
|
19 |
20 |
22 |
23 |
24 |
|
|||||||||||
|
invasion of large blood vessels |
No invasion |
0 |
0 |
0 |
0 |
0 |
7 |
|||||||||
|
With invasion |
2 |
2 |
1 |
1 |
2 |
63 |
||||||||||
|
Total |
2 |
2 |
1 |
1 |
2 |
70 |
||||||||||
|
Symmetric measures |
|
|||||||||||||||
|
Variables |
Significance |
Approximate significance
|
||||||||||||||
|
Nominal/nominal |
Phi |
0.630 |
0.066
|
|||||||||||||
|
Cramer's V |
0.630 |
0.066 |
||||||||||||||
|
Number of admissible observations |
70 |
|||||||||||||||
Liver lesion volume: The influence of liver lesion volume on invasion into large blood vessels in patients with AE was analyzed. The data presented in the cross-sectional Table 6 demonstrate the following: among patients without invasion (7 patients), only 1 patient had 1 liver sector involved, 5 patients had 2 sectors, and 1 patient had 3 sectors involved. While in
the group of patients with invasion (63 patients), the distribution of lesion volumes is more variable: 15 patients had 2 sectors involved, 25 patients had 3 sectors, and 17 patients had 4 liver sectors involved. Cramer's V of 0.346 indicates that there is a moderate correlation between liver lesion volume and invasion into large blood vessels.
However, the value of p = 0.078 indicates that this correlation is not statistically significant at the 0.05 level.
Thus, although there is a trend toward greater liver lesion volume in patients with invasion, the statistical significance of this relationship is not confirmed with a high degree of certainty. These data suggest that as the volume of liver lesion increases, the probability of invasion into large blood vessels increases
|
Table 6. Relationship between lesion volume and vascular invasion |
|||||||
|
Cross table |
|||||||
|
Count |
|||||||
|
Variable |
Total lesion volume of liver, sectors |
Total |
|||||
|
Only S1 is affected |
1 sector is involved |
2 sectors of are invloved |
3 sectors are involved |
4 sectors are involved |
|||
|
Invasion of large blood vessels |
No invasion |
0 |
1 |
5 |
1 |
0 |
7 |
|
With invasion |
1 |
5 |
15 |
25 |
17 |
63 |
|
|
Total |
1 |
6 |
20 |
26 |
17 |
70 |
|
|
Symmetric measures |
|||||||
|
Significance |
Approximate significance |
||||||
|
Nominal/nominal |
Phi |
0.346 |
0.078 |
||||
|
Cramer's V |
0.346 |
0.078 |
|||||
|
Number of admissible observations |
70
|
|
|||||
Discussion
The study aimed to investigate factors associated with vascular invasion in patients with hepatic AE. Among the various analyzed factors, the number of foci and the size of the largest focus showed the most significant associations with vascular invasion. The statistical analysis revealed that patients with multiple foci and larger foci (>10 cm) were more likely to experience vascular invasion. Specifically, the number of foci demonstrated moderate significance (Cramer's V = 0.404, p = 0.022), and the size of foci approached statistical significance (Cramer's V = 0.630, p = 0.066). In comparison to other factors like age, sex, foci morphology (according to AEUC classification), and liver sectors involvement showed less significant correlation with vascular invasion.
The findings of this study with comparison to previous research that has suggested the number of foci and the size of the foci as key factors in the development of vascular invasion in hepatic AE. Similar studies have
shown that larger lesions are more prone to invasion, likely due to their ability to extend into surrounding structures, including blood vessels. However, the present study contributes by emphasizing that a focus size greater than 10 cm is of particular concern, potentially marking a threshold for increased vascular invasion risk.
Additionally, previous studies have often indicated mixed results regarding the role of sex and age in predicting vascular invasion. In this study, no significant relationship was found between sex and vascular invasion (Cramer's V = 0.201, p = 0.092), and although there was a traceable trend concerning age, it did not reach statistical significance (Cramer's V = 0.768, p = 0.288). This is consistent with other research that has shown variability in the impact of demographic factors on AE progression, suggesting that vascular invasion may be more dependent on lesion characteristics rather than host factors like age or sex.
Among patients with vascular invasion, females predominated (33 vs. 30 males). However, no statistically significant association between sex and blood vessel invasion was found (Cramer's V = 0.201, p = 0.092). Most patients with vascular invasion had ages ranging from 20 to 45 years. Still, the value of Cramer's V = 0.768 and p = 0.288 suggest that age is not directly related to the risk of vascular invasion.
Probably, it is necessary to continue dynamic observation of changes depending on age, as a certain age trend is traceable. All the above-mentioned suggests that sex and age are not the key predictors of the risk of vascular invasion in liver AE.
The number of foci according to the study was the most significant risk factor for blood vessel invasion (Cramer's V = 0.404, p = 0.022), statistical significance was moderate. The correlation suggests that the presence of more than one foci is associated with an increased risk of vascular invasion. Foci size also had moderate statistical significance (Cramer's V = 0.630, p = 0.066), indicating an increased likelihood of invasion in foci larger than 10 cm. Which further emphasizes the importance of early diagnosis of liver AE. Morphologic type of foci according to the AEUC classification showed a weak association (Cramer's V = 0.184, p = 0.666). Foci of types 3 and 4 more often sprouted into vessels (30 and 18 patients, respectively) with no statistically significant association. Patients with invasion into vessels were more likely to have liver sector 2 and 3 lesions (15 and 25 patients, respectively), Cramer's V = 0.346 and p = 0.078 did not reach the threshold for statistical significance; however, an increase in liver lesion volume was associated with a higher likelihood of invasion.
Thus, our analysis shows that in patients with first-diagnosed hepatic AE, the most significant predictors of invasion into large vessels are the number of foci and the size of the largest foci. These factors have a moderate statistical correlation, whereas the AEUC foci type, lesion volume, sex, and age have less influence on the invasion development.
Study limitations
While the findings are informative, several limitations must be considered. First, the relatively small sample size (39 females vs. 31 males) may limit the generalizability of the results. Larger, multicenter studies would help confirm whether sex and age have any real role in predicting vascular invasion or if the observed lack of association is a result of statistical power issues.
Second, the study primarily focused on the morphological characteristics of AE foci (number and size) and did not correlate molecular or histopathological factors that could provide further insights into the mechanisms of vascular invasion. For instance, understanding the molecular pathways that enable AE lesions to invade vessels could improve prediction models.
Finally, the cross-sectional nature of the study limits the ability to establish causal relationships. Longitudinal studies would be beneficial to track changes in lesion behavior over time and assess how these factors evolve in relation to vascular invasion.
Future research directions
Given the findings and limitations, several avenues for future research could be explored:
1. Longitudinal Studies: To better understand the progression of AE and the risk factors for vascular invasion, longitudinal studies should trace patients over time. This could provide insights into whether lesion size and number consistently predict vascular invasion or if other factors, such as host immune response or genetic predispositions, play a role.
2. Molecular and Histopathological Studies: Future research could examine the molecular mechanisms causing vascular invasion in AE. Identifying specific markers or pathways involved in invasion could lead to the development of targeted treatment to prevent the complication.
3. Larger, Multicenter Cohorts: Expanding the study to include more participants from multiple centers would provide a more robust analysis of the risk factors for vascular invasion, particularly in relation to sex, age, and liver sector involvement. Additionally, this would allow for more accurate statistical analysis and the potential identification of new risk factors.
4. Imaging Techniques and Early Diagnosis: Given the significant association between foci size and number with vascular invasion, future research could explore advanced imaging techniques like contrast – enhanced ultrasound and MRI for early detection and assessment of these characteristics. This techniques will help identify at-risk patients earlier and facilitating timely intervention.
Conclusion
The invasion of parasitic nodules into large blood vessels in patients with first diagnosed hepatic alveolar echinococcosis does not depend directly on sex, age, type of focus according to AEUC. It can be stated that with a high probability invasion of pathologic focus into the vascular wall is associated with an increase in the volume of liver lesion. The most significant predictors of invasion into large vessels are the number of foci and the size of the largest foci.
Ethics: Ethical approval was obtained from ethical committee of I. K. Akhunbaev Kyrgyz State Medical Academy. Informed consent was obtained from all patients for diagnostic procedures.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: I.A.B., A.I.K., K.O., B.K.B., K.I., A.O., R.K.S.,
and S.N.I equally contributed to the study and preparation of manuscript, fulfilled all authorship criteria
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Contact authors. Fair use, acknowledgement of authors and sources, and ethics rules apply.
References
| 1.Meinel TR, Gottstein B, Geib V, Keel MJ, Biral R, Mohaupt M, et al. Vertebral alveolar echinococcosis-a case report, systematic analysis, and review of the literature. Lancet Infect Dis 2018; 18: e87-e98. doi: 10.1016/S1473-3099(17)30335-3 https://doi.org/10.1016/S1473-3099(17)30335-3 PMid:28807628 |
||||
| 2. Umhang G, Possenti A, Colamesta V, d'Aguanno S, La Torre G, Boue F, et al. A systematic review and meta-analysis on anthelmintic control programs for Echinococcus multilocularis in wild and domestic carnivores. Food Waterborne Parasitol 2019; 15: e00042. doi: 10.1016/j.fawpar.2019.e00042. https://doi.org/10.1016/j.fawpar.2019.e00042 PMid:32095614 PMCid:PMC7034083 |
||||
| 3. Paternoster G, Boo G, Wang C, Minbaeva G, Usubalieva J, Raimkulov KM, et al. Epidemic cystic and alveolar echinococcosis in Kyrgyzstan: an analysis of national surveillance data. Lancet Glob Health 2020; 8: e603-11. doi: 10.1016/S2214-109X(20)30038-3 https://doi.org/10.1016/S2214-109X(20)30038-3 PMid:32199126 |
||||
| 4.Grüner B, Schmidberger J, Drews O, Kratzer W, Gräter T. Imaging in alveolar echinococcosis Comparison of Echinococcus multilocularis classification for computed-tomography (EMUC-CT) and ultrasonography (EMUC-US). Radiol Infect Dis 2017; 4: 70-7. doi: 10.1016/J.JRID.2017.05.001 https://doi.org/10.1016/j.jrid.2017.05.001 |
||||
| 5. Voskanyan SE, Bashkov AN, Karmazanovsky GG, Naydenov EV, Ionova EA. Planning principles for radical surgical intervention for liver alveococcosis based on computed and magnetic resonance imaging. Ann HPB Surg 2020; 25: 100-12. doi: 10.16931/1995-5464.20202100-112 https://doi.org/10.16931/1995-5464.20202100-112 |
||||
| 6. Bebezov BK, Bebezov KhS, Umetaliev TM, Mamashev ND, Surov EA, Ryspekov BZ, et al. Advanced liver resections in alveococcosis. Vestnik KRSU 2022; 22: 23-9. doi: 10.36979/1694-500X-2022-22-1-23-29 https://doi.org/10.36979/1694-500X-2022-22-1-23-29 |
||||
| 7. Omorov RA, Aitbayev SA, Kanietov AK, Abdiev AA. Results of surgical treatment of patients with hepatic alveococcosis. Annals Surg Heaptol 2018; 23: 71-9.. doi: 10.16931/1995-5464.2018-1-74-79 https://doi.org/10.16931/1995-5464.2018-1-74-79 |
||||
| 8. Graeter T, Schmidberger j. Stage-oriented CT classification and intermodal evolution model in hepatic alveolar echinococcosis. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr 2022; 194: 532-44. doi: 10.1055/a-1710-3669 https://doi.org/10.1055/a-1710-3669 PMid:35081647 PMCid:PMC9133419 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

