Cryoablation of pulmonary veins in a patient with an atrial septal occluder and atrial fibrillation: A case report
CASE REPORT
Cryoablation of pulmonary veins in a patient with an atrial septal occluder and atrial fibrillation: A case report
Article Summary
- DOI: 10.24969/hvt.2025.555
- CARDIOVASCULAR DISEASES
- Published: 21/03/2025
- Received: 17/01/2025
- Revised: 19/02/2025
- Accepted: 20/02/2025
- Views: 3068
- Downloads: 2194
- Keywords: Cryoballoon isolation, atrial septal defect, occluder, atrial fibrillation, ablation, case report
Address for Correspondence: Zhansaya Yerkhanova, Department of Cardiology, Arrhythmology, Medical Center Hospital of the President’s Affairs Administration of the Republic of Kazakhstan, Astana, 010000, Kazakhstan
Email: 11lzhansayae@gmail.com Mobile: +7 776 110 25 26
Ayan Abdrakhmanov1,2, Zhansaya Yerkhanova1*, Zhanasyl Suleymen2, Rano Kirkimbaeva2
1Department of Cardiology, Arrhythmology, Medical Center Hospital of the President’s Affairs Administration of the Republic of Kazakhstan, Astana, Kazakhstan
2Department of Cardiology, Astana Medical University, Astana, Kazakhstan
Abstract
Objective: An atrial septal defect (ASD) is one of the most common congenital heart defects and is associated with an increased risk of atrial arrhythmias. Closure of the ASD reduces this risk; however, when the correction is performed later in life, there is a higher risk of arrhythmias compared to those who undergo the procedure at an earlier age. Cryoballoon ablation of the pulmonary vein orifices is an effective and widely available treatment for symptomatic atrial fibrillation (AF) that is refractory to drugs. Given that this procedure requires access to the left atrium, the presence of an implanted occluder may pose several challenges during surgical intervention. This case study explores the safety of using interventional treatment in patients with an atrial septal occluder for AF.
We demonstrate an effective and widely available surgical treatment for symptomatic AF refractory to drugs patient with an atrial septal occluder.
Case presentation: We report the case of a 57-year-old woman with an implanted atrial septal occluder who underwent cryoablation for drug-refractory and symptomatic paroxysmal AF. The "Occlutech Figulla Flex II" transseptal occluder was implanted in February 2015 due to an ASD. We used cardiac computed tomography (CT) with contrast to visualize the size and position of the occluder in the atrial septum and determine the puncture area. A single transeptal puncture was performed infero-posterior to the occluder under X-ray control, allowing cryoablation of the pulmonary veins. After the procedure, X-ray and transthoracic echocardiography showed an unchanged device position. After 6 months, the patient had no episodes of AF.
Conclusion: In this case, cryoballoon ablation for AF in a patient with an atrial septal occluder was feasible, safe, and effective. The use of cardiac CT with contrast in the preoperative period helps visualize the size and position of the occluder in the atrial septum, determine the size of the native septum, and outline the proposed puncture site. However, further studies are needed to determine the generalizability of these findings.
Key words: Cryoballoon isolation, atrial septal defect, occluder, atrial fibrillation, ablation, case report
Introduction
![]() Atrial septal defect (ASD) is a common congenital heart disease with a birth prevalence of 2.5 per 1,000 live births, and 25% to 30% of cases occur in adulthood (1, 2). The main hemodynamic abnormalities in the natural course of an atrial septal defect include prolonged blood flow from the left to the right atrium, resulting in volume overload of the right atrium and right ventricle, and subsequent changes in the electrophysiological properties of both atria (3). These changes increase the risk of secondary cardiac rhythm disturbances. Paroxysms of tachyarrhythmia worsen cardiac hemodynamics and contribute to the progression of heart failure (4). Atrial fibrillation (AF) is among the most frequent types of rhythm disturbances in adult patients with ASDs (5).
Atrial septal defect (ASD) is a common congenital heart disease with a birth prevalence of 2.5 per 1,000 live births, and 25% to 30% of cases occur in adulthood (1, 2). The main hemodynamic abnormalities in the natural course of an atrial septal defect include prolonged blood flow from the left to the right atrium, resulting in volume overload of the right atrium and right ventricle, and subsequent changes in the electrophysiological properties of both atria (3). These changes increase the risk of secondary cardiac rhythm disturbances. Paroxysms of tachyarrhythmia worsen cardiac hemodynamics and contribute to the progression of heart failure (4). Atrial fibrillation (AF) is among the most frequent types of rhythm disturbances in adult patients with ASDs (5).
Given that this procedure requires access to the left atrium, the presence of an implanted occluder can pose several challenges during intervention. The presence of an atrial septal occluder (ASO) complicates and increases the risk of transseptal puncture, posing a significant challenge to the broader use of catheter ablation in patients with a history of ASD closure. Many clinics and physicians avoid endocardial pulmonary vein ablation for patients with this pathology due to the difficulty of puncturing the atrial septum, despite the high symptom burden in these patients.
The aim of this case report is to evaluate the safety and feasibility of cryoballoon ablation for atrial fibrillation in a patient with a previously implanted atrial septal occluder.
Case report
A 57-year-old woman was admitted to the hospital due to progressive shortness of breath and frequent episodes of palpitations, despite taking antiarrhythmic drugs. Her condition had significantly deteriorated, manifesting as increasing weakness and fatigue. An electrocardiogram at admission showed AF with a heart rate of 115 beats per minute. According to her history, she had been experiencing tachycardia attacks since the age of 35. In 2015, she was diagnosed with an ASD, which was corrected the same year through the implantation of an Occlutech Figulla Flex II occluder (15 mm size). Despite previously taking antiarrhythmic drugs from class IC (propafenone) and class III (amiodarone), the therapy was ineffective, and she continued to experience poorly tolerated heart palpitations in daily life.
Transthoracic echocardiography (TT Echo-CG) revealed that both the right and left atria were within the normal range, the occluder in the atrial septum was correctly implanted, both discs were fully opened, and there were no blood shunts. Transesophageal echocardiography showed no blood clots in the heart chambers. Cardiac computed tomography (CT) with contrast revealed atherosclerotic changes in the left anterior descending artery without significant lumen narrowing. The patient's condition was noted as post-endovascular closure of the ASD.
Based on the analysis of the anatomic structure, the decision was reached to do transseptal puncture on the native septum.
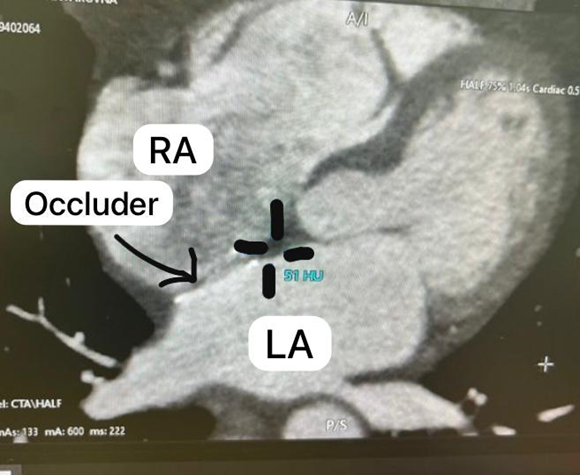
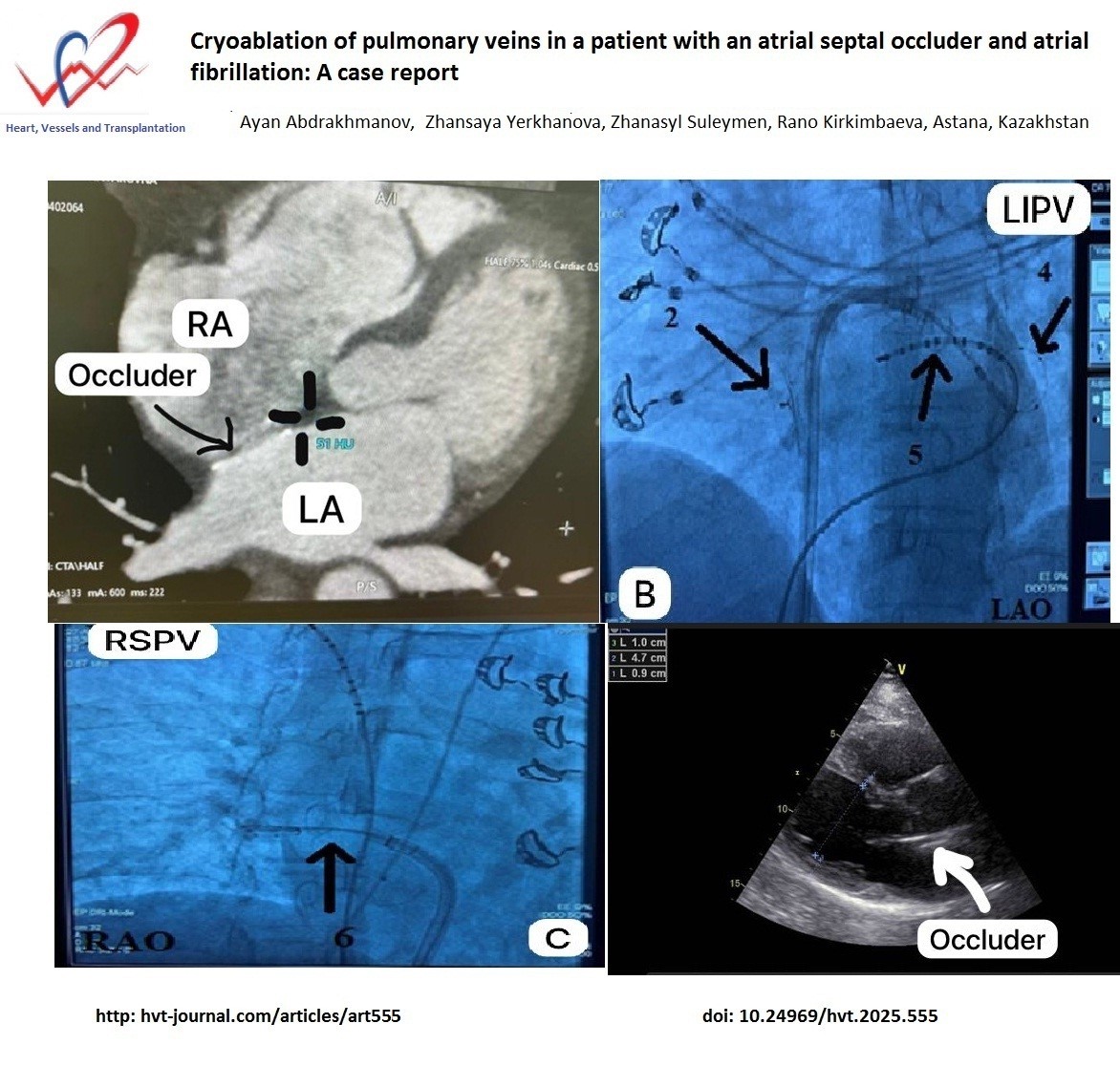
Figure 1. Axial slice of computed tomography at the level of the left atrium and interatrial septum. The marking indicates the area of the native interatrial septum selected for transseptal puncture
We discovered that the native septum between the left atrium (LA) and right atrium is appropriate for the transseptal puncture; the puncture was made through the native septum inferior to the ASO.
The ablation strategy for patients with paroxysmal AF was circumferential pulmonary vein (PV) isolation:
1. Given the implanted occluder in the atrial septum, cryoballoon isolation of the PVs was chosen to minimize access to the LA. The patient was taken to the catheterization laboratory. Initial atrial stimulation showed frequent atrial extrasystoles with a heart rate of 64 bpm. Under local anesthesia, the right common femoral vein was punctured twice. A 10-pole electrode was placed in the coronary sinus, and a Swartz introducer was positioned in the right atrium. Transseptal puncture was performed using a transseptal needle under fluoroscopy, and 10,000 IU of heparin was administered intravenously. Then the needle was advanced alongside the device across the native septum.
2. After air embolism prevention, the Swartz introducer was replaced with a FlexCath-controlled introducer. A 28mm ArcticFrontAdvance balloon catheter was advanced into the LA. The left superior pulmonary vein (LSPV) was cannulated with the Achieve diagnostic catheter, and cryoablation was performed for 240 seconds, reducing the balloon temperature to -48°C, eliminating the PV endogram. The left inferior pulmonary vein (LIPV) was cannulated, and cryoablation was conducted for 240 seconds, lowering the balloon temperature to -50°C, with PV endogram elimination.
3. A diagnostic catheter was placed at the confluence of the right subclavian vein and superior vena cava SVC to stimulate the phrenic nerve, preventing paresis. The right inferior pulmonary vein (RIPV) was cannulated, and cryoablation was performed for 240 seconds, reaching -46°C, eliminating the PV endogram. The right superior pulmonary vein (RSPV) was cannulated, and cryoablation was carried out for 240 seconds, reducing the balloon temperature to -56°C, with PV endogram elimination. Pulmonary vein isolation was confirmed using the Achieve catheter.
4. The procedure was completed, electrodes were removed, and hemostasis was achieved.
We discovered that the native septum between the left atrium and right atrium is appropriate for the transseptal puncture; the puncture was made through the native septum inferior to the ASO.
There were no technical difficulties with balloon positioning. The procedure lasted 60 minutes, with 10 minutes of fluoroscopy. No complications were observed post-intervention. Transthoracic echocardiography at 2 hours, and on the 1st and 2nd days after the procedure, showed no signs of occluder damage or blood shunts. The patient was discharged on the 2nd day.
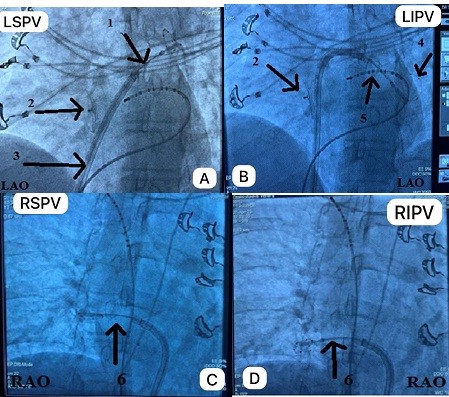
Figure 2. Cryotherapy in the left superior (a), left inferior (b), right superior (c), and right inferior (d) pulmonary veins. 1 – Balloon, 2 - Occluder shadow, 3 - Delivery catheter, 4 - Diagnostic catheter (Achieve), 5 - 10-polar catheter
Figure 3. Transthoracic echocardiography after ablation. Parasternal long axis view demonstrates functioning occluder
Follow-up
Post-procedure, the patient was placed on anticoagulation therapy with rivaroxaban to prevent thromboembolic events for 3 months. Antiarrhythmic medications, including amiodarone, were also continued for 3 months. The postoperative period was uneventful. At 3- and 6-month follow-up, the patient reported no palpitations, and instrumental studies showed no evidence of rhythm or conduction disorders.
Discussion
Our case demonstrated that cryoablation of AF in a patient with ASO is safe and effective, with no damage to occluder after procedure and complications.
Several cases describe the outcomes of interventions for atrial arrhythmias in patients with previously implanted occluders for atrial septal defects. However, studies are limited due to the difficulty of access and the complexity of the procedure, leading to fewer operations on patients with occluders and a history of arrhythmias.
According to Cai-Hua Sang (7), a study included 16 patients with pharmacoresistant AF (10 paroxysmal and 6 persistent) who had a previously implanted occluder. Notably, the study did not use transesophageal or intracardiac echocardiography. Instead, right atrial contrast was used to assess the atrial septum and determine the position of the occluder relative to major vessels. If passage through the native septum was not possible, balloon dilation of the occluder was performed. In cases of paroxysmal AF, circular pulmonary vein isolation was the strategy, while in persistent AF, additional linear ablation was performed. Transseptal access was achieved through the native septum in 11 patients (Group A) and through the occluder with balloon dilation in 5 patients (Group B). Circular pulmonary vein isolation was successful in all patients, and linear ablation was achieved in all persistent AF cases, except for one patient who did not achieve mitral isthmus blockage. No atrial bypass grafting was detected by transthoracic echocardiography after 3 months. During a follow-up period of 16 ± 6 months, sinus rhythm was maintained in 12 out of 16 patients, with no serious complications.
Conclusion
In this case, cryoballoon ablation for AF in a patient with an atrial septal occluder was feasible, safe, and effective. The use of cardiac CT with contrast during the preoperative period helps visualize the size and position of the occluder in the atrial septum, allows for determining the size of the native septum, and provides a preliminary outline of the site for the proposed puncture. Additionally, due to the lack of tactile feedback during transseptal access with an atrial septal occluder, intracardiac or transesophageal echocardiography is recommended for better visualization and a safer transition to the left atrium. However, further studies are needed to assess the generalizability of these findings.
Ethics: Informed consent was obtained from patient for all procedures.
Peer-review: Internal
Conflict of interest: None to declare
Authorship: A.A., Zh.Y., Zh.S. and R.K. equally contributed to case management and preparation of manuscript
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Do not apply
References
| 1.van der Linde D, Konings EEM, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJM, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta‐analysis. J Am Coll Cardiol 2011; 58: 2241-7. doi: 10.1016/j.jacc.2011.08.025 https://doi.org/10.1016/j.jacc.2011.08.025 PMid:22078432 |
||||
| 2 Brida M, Chessa M, Celermajer D, Li W, Geva T, Khairy P, et al. . Atrial septal defect in adulthood: a new paradigm for congenital heart disease. Eur Heart J 2022; 43: 2660-71. doi: 10.1093/eurheartj/ehab646 https://doi.org/10.1093/eurheartj/ehab646 PMid:34535989 |
||||
| 3.Nyboe C, Karunanithi Z, Nielsen‐Kudsk JE, Hjortdal VE. Long‐term mortality in patients with atrial septal defect: a nationwide cohort‐study. Eur Heart J 2018; 39: 993-8. doi: 10.1093/eurheartj/ehx687 https://doi.org/10.1093/eurheartj/ehx687 PMid:29211856 PMCid:PMC6037065 |
||||
| 4.Dolgner SJ, Steinberg ZL, Jones TK, Reisman M, Buber J. Stroke in patients with secundum atrial septal defect and sequelae after transcatheter closure. Heart 2021; 107: 1875-80. doi: 10.1136/heartjnl-2021-319050 https://doi.org/10.1136/heartjnl-2021-319050 PMid:34380660 |
||||
| 5.Blake GE, Lakkireddy D. Atrial septal defect and atrial fibrillation: The known and unknown. J Atr Fibrill 2008;1: 45. doi: 10.4022/jafib.45 https://doi.org/10.4022/jafib.v1i1.390 |
||||
| 6.Gatzoulis MA, Freeman MA, Siu SC, Gary D, Webb GD, Harris L. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med 1999; 340: 839-46. doi: 10.1056/NEJM19990318340110 https://doi.org/10.1056/NEJM199903183401103 PMid:10080846 |
||||
| 7 Sang CH, Dong JZ, Long DY, Yu RH, Bai R, Salim M, et al. Transseptal puncture and catheter ablation of atrial fibrillation in patients with atrial septal occluder: initial experience of a single centre. Europace 2018; 20: 1468-74. doi:10.1093/europace/eux282 https://doi.org/10.1093/europace/eux282 PMid:29106529 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

