Diagnostic imaging modalities in the study of hepatic alveolar echinococcosis: A review of literature
REVIEW
Diagnostic imaging modalities in the study of hepatic alveolar echinococcosis: A review of literature
Article Summary
- DOI: 10.24969/hvt.2025.558
- CARDIOVASCULAR DISEASES
- Published: 05/04/2025
- Received: 19/11/2024
- Revised: 28/01/2025
- Accepted: 28/01/2025
- Views: 5813
- Downloads: 3233
- Keywords: Alveococcosis, hepatic alveolar echinococcosis, Echinococcus multilocularis, liver, metastasis, ultrasonography, computed tomography, magnetic resonance imaging, positron-emission tomography
Address for Correspondence: Prabha K C, Radiology Department, I.K. Akhunbaev Kyrgyz State Medical Academy;
Email: keucuprabha12@gmail.com Phone: +996228035497
1aIliar Baudinov, 1aAliya Kadyrova, 1aKubat Ibraimov, 1a*Prabha K C,1bBakhadyr Bebezov, 1cSezdbek Aitbaev,
2Tilek Umetaliev, 1d Kursanbek Raimkulov
1aDepartment of Radiology, 1bDepartment of Innovative Surgical Technologies, 1cDepartment of Faculty Surgery the named after I.K. Ahunbaev, and 1dDepartment of Medical Biology, Genetics and Parasitology, I.K. Akhunbaev Kyrgyz state Medical academy, Akhunbaev str., 720020, Bishkek, Kyrgyz Republic
2Department of Hospital Surgery, Faculty of Medicine, Kyrgyz-Russian Slavic University, Kievskaya St. 44, 720065, Bishkek, Kyrgyz Republic
Abstract
Objective: This review article aims to summarize the main imaging modalities employed in the diagnosis of hepatic alveolar echinococcosis (HAE) and provide radiologists with an overview of the most relevant diagnostic approaches.
Methods: A literature review was conducted, selecting the most relevant articles from the past 5–10 years. The search was performed in PubMed, MDPI, Scopus, Web of Science eLIBRARY.RU, using the keywords alveococcosis, hepatic alveolar echinococcosis, echinococcus multilocularis, liver, metastasis, USG, CT, MRI, positron-emission tomography, imaging, diagnosis. Illustrative materials presented in the article originate from the authors' personal archive.
Results: Hepatic alveolar echinococcosis (HAE) is a chronic parasitic disease characterized by an infiltrative growth pattern, often mimicking malignant liver tumors. Its ability to invade adjacent structures and disseminate to distant organs necessitates a comprehensive imaging approach for accurate diagnosis and staging.
Ultrasound (US) serves as the primary screening tool for HAE, particularly in remote endemic regions where advanced imaging modalities may be unavailable. Its accessibility and real-time imaging capabilities allow for the initial detection of hepatic lesions. However, due to its limited ability to assess vascular and biliary involvement, further imaging is required for precise disease evaluation.
Contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are the cornerstone imaging techniques for assessing both hepatic and extrahepatic disease extension. CT provides detailed anatomical visualization of the liver parenchyma and is essential for evaluating lesion size, vascular encroachment, and potential metastatic spread. MRI, particularly magnetic resonance cholangiopancreatography (MRCP), is superior in delineating biliary tree involvement, facilitating preoperative planning and surgical decision-making.
To standardize disease staging, the World Health Organization (WHO) has introduced the PNM classification system, which parallels the oncological TNM framework. This classification system enables a systematic assessment of the parasite burden within the liver (P), its invasion of adjacent structures (N), and the presence of distant metastases (M), thereby guiding therapeutic strategies.
Conclusion: Radiologists should be aware of the specific imaging features of HAE to avoid misdiagnosis as a malignancy. A multimodal imaging approach is recommended to ensure accurate diagnosis and staging.
Key words: Alveococcosis, hepatic alveolar echinococcosis, Echinococcus multilocularis, liver, metastasis, ultrasonography, computed tomography, magnetic resonance imaging, positron-emission tomography
Introduction
Hepatic alveolar echinococcosis (HAE) is a chronic zoonotic infection caused by the pseudo-tumoral intrahepatic development of the larval stage of the tapeworm Echinococcus multilocularis, which mainly affects the liver (1, 2). Until the 19th century, the disease was described as one of the forms of liver tumors, and only in 1856, R. Virchow disclosed its true nature (3).
The natural reservoirs for alveococcus are mainly found in Europe (Poland, Germany, Austria, Switzerland), Russia, Asia (Kyrgyzstan, Kazakhstan, China, Japan), and North America (4, 5).
Graphical abstract
Every year around 11 400 to 29 600 people are infected with HAE, with a fatal outcome in 17 000 people (6). According to the World Health Organization (WHO), it causes 19,300 deaths annually (6).
Alveococcosis is defined as ‘parasitic cancer’, because of its infiltrating nature (7). This is actually appropriate given that the disease has an infiltrating growth pattern and dissemination of larvae from the primary focus to surrounding tissues and organs (8). After a parasitic node grows to a substantial size, it affects the bile and lymphatic ducts or blood vessels, which is a characteristic of malignancy (9-11). Metastases occur when the parasites spread via a hematogenous or lymphatic route secondary to the liver, known as intrahepatic metastases, or when they disseminate to the regional and retroperitoneal lymph nodes, referred to as extrahepatic metastases(10-13). They can expand into neighboring tissues and organs (kidney, bones, diaphragm, further into the pleural cavity and lung, resulting in broncho-biliary fistula), and give distant metastases to the lungs (4.7-20%), brain (1-3.3%), bones, peritoneum, and other organs (14-16). According to studies published, distant metastases of patients range from 6.3% to 34% of cases (17- 22).
The spread of the alveolar node through the diaphragm and vascular bed, primarily through the inferior vena cava, can reach the pleural cavity, mediastinum, right side of the heart, and muscles as well (23). With the addition of secondary infection, cholangitis, liver abscesses, suppuration and disintegration of nodes occur (24. 25).
Ultrasound (US) serves as the primary screening tool for HAE, particularly in remote endemic regions where advanced imaging modalities may be unavailable (26). Computed tomography (CT) with bolus contrast is essential for all patients with hepatic alveococcosis, as it provides valuable diagnostic information (27, 28). Implementing CT volumetry is highly recommended for accurately determining the residual volume of the liver parenchyma (29). Magnetic resonance imaging (MRI) is an excellent tool for evaluating vascular and biliary tract involvement (30). Currently, MR cholangiopancreatography has largely replaced percutaneous cholangiography in assessing the relationship between HAE lesions and the biliary tree (31). Without appropriate treatment, parasitic infection leads to death in 95% of cases, typically from hepatic failure or cachexia within 5 to 10 years (32).
HAE is diagnosed based on an epidemiological history, physical examination, radiological examination, laboratory tests, and histopathological examination (33, 34). In 1996, based on radiation imaging methods, the WHO Informal Working Group of Experts on Echinococcosis (WHO-IWGE) developed a clinical classification known as the PNM system (P – Parasite, N – Neighboring organs, M – Metastasis), similar to the TNM oncological classification (T – Tumor, N – Nodes, M – Metastasis), to describe the anatomical distribution of the parasitic process (1). In the PNM system, category P evaluates the dissemination of parasites in the liver and the involvement of its tubular structures. Category N accesses the involvement of adjacent organs and anatomical structures. Category M assesses the presence of distant metastases (Table 1) (35).
|
Table 1. PNM classification of human hepatic alveolar echinococcosis |
|
|
Classified according to the data received |
|
|
P: Hepatic localization of the primary lesion |
|
|
PX |
Primary lesion cannot be assessed |
|
P0 |
No detectable liver lesions |
|
P1 |
Peripheral lesions without proximal vascular and/or biliary involvement |
|
P2 |
Central lesions with proximal vascular and/or biliary involvement of one lobe |
|
P3 |
Central lesions with hilar vascular and biliary involvement of both lobes and/or with involvement of two hepatic veins |
|
P4 |
Any lesion with extension along large vessels (IVC, portal vein, hepatic veins, hepatic arteries) and the biliary tree |
|
N: Extra hepatic involvement of neighboring organs or tissues involving diaphragm, pleura, lungs, pericardium, heart, adrenal glands, kidneys, pancreas, liver ligaments, regional lymph nodes, abdominal walls, muscles, skin, bones |
|
|
NX |
Cannot be evaluated |
|
N0 |
No regional involvement |
|
N1 |
Regional involvement of contiguous organs or tissues |
|
M: Absence or presence of distant metastases (lungs, non-regional lymph nodes, CNS, orbits, bones, skin, muscles, kidneys, abdomen, retroperitoneum) |
|
|
MX |
Distant organ metastases not completely evaluated |
|
M0 |
No distant metastases. |
|
M1 |
Distant metastases present |
|
CNS- central nervous system, PNM – parasite, node, metastasis Reproduced from ref. 35 under CC-BY license |
|
For staging of alveolar echinococcosis, the PNM classification is used (Table 2).
|
TabLe 2. Staging of alveolar echinococcosis based on PNM classification |
|
|
Stage of alveolar echinococcosis |
PNM classification |
|
Stage I |
P1 N0 M0 |
|
Stage II |
P2 N0 M0 |
|
Stage IIIa |
P3 N0 M0 |
|
Stage IIIb |
P1– 3 N1 M0 |
|
P4 N0 M0 |
|
|
Stage IV |
P4 N1 M0 |
|
Any P Any N and/or M1 |
|
|
PNM – parasite, node, metastasis Reproduced from ref. 35 under CC-BY license |
|
The accurate diagnosis of hepatic alveolar echinococcosis (HAE) is crucial for determining the appropriate treatment strategy and avoiding misdiagnosis as a malignant liver tumor (36). Since HAE exhibits an infiltrative growth pattern and can spread beyond the liver, the selection of imaging modalities must be carefully considered to ensure a comprehensive assessment of the disease (37).
Methods
A systematic literature search was conducted using the databases PubMed, Scopus, Web of Science. The search covered articles published between 2013–2023 and included studies in English and Russian. The following keywords were used: "hepatic alveolar echinococcosis", "metastasis", "imaging modalities", "CT", "MRI", "WHO classification". Studies that did not provide imaging findings or were unrelated to hepatic alveolar echinococcosis were excluded.
Imaging modalities in hepatic alveolar echinococcosis
Some commonly used imaging modalities for the diagnosis and follow-up of HAE include ultrasonography (USG), CT, and MRI (38).
Ultrasound
Ultrasonography is the most effective method for the initial diagnosis and dynamic monitoring of alveococcosis (39, 40). Its main advantage is accessibility, which allows its use in endemic 'hot spots,' significantly reducing the time for primary diagnosis and enabling earlier treatment (41). However, specific details regarding the ultrasonographic semiotics of HAE are scarce in the literature and are found in only limited sources due to the peculiarities of the geographical endemicity of the infection (42, 43). Thus, in B-mode ultrasound (Brightness mode), five types of parasitic nodes are classified (EMUC-US: Echinococcus multilocularis sonomorphological classification): hailstorm, pseudocystic, hemangioma-like, ossification, and metastasis-like (44). Each of these five types has its own characteristic ultrasound patterns (45).
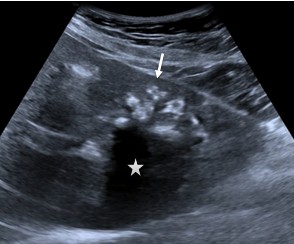
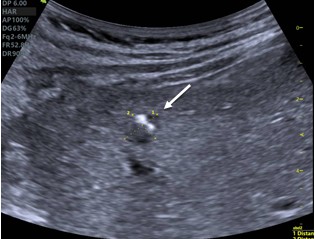
Type 1. Hailstorm is characterized by the presence of hyperechoic structures with/without dorsal acoustic shadowing, indistinct uneven contours, and heterogeneous structure (Fig. 1).
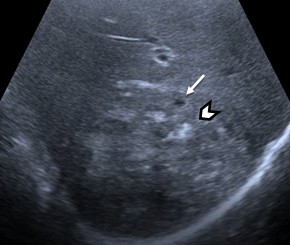
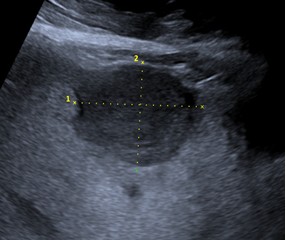
Type 2. Pseudocystic are mainly characterized by a pseudo-cystic pattern with no clear contours, a hypo- or an-echoic heterogeneous structure, often with heterogeneous central zone due to hyperechoic inclusions, with a hyperechoic halo (rim), avascular in power or color Doppler imaging. These lesions may be larger than 10 mm. They can develop from the primary hailstorm type, and during initial presentation and scanning of asymptomatic patients, the first two types can be detected simultaneously, forming the “typical pattern of HAE”. Sometimes, sizes that can already occupy the entire lobe of the liver (Fig. 2).
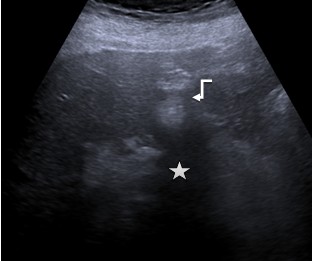
Type 3. Hemangiomas-like: these lesions are difficult to distinguish from atypical hemangiomas (eg, that are partially thrombosed); they often create a major diagnostic dilemma. The lesions are characterized by increased echogenicity, and range from indistinct/clearly defined tumors of a heterogeneous structure, relatively echogenic in relation to the liver parenchyma to a pronounced hyperechoic pattern of a homogeneous structure (Fig. 3).
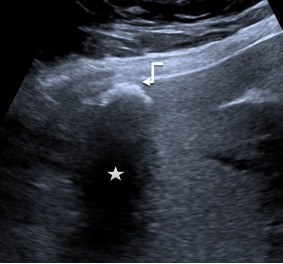
Type 4 Ossification: this pattern is mostly single or multifocal hyperechoic structure with strong dorsal acoustic shadowing. In the differential diagnosis, it is often difficult to distinguish from inflammatory structures or metastases of various carcinomas. Large foci are rare (Fig. 4).
|
|
|
|
|
Figure 1. “Hailstorm”: a heterogeneous structure, with the presence of hyperechoic structures (arrow) with a dorsal acoustic shadowing (star) and clear uneven contours |
Figure 2. Typical pattern of hepatic alveococcosis, a combination of the first two types, “hailstorm” (arrow head) and “pseudocysts” (arrow) |
|
|
|
|
|
|
Figure 3. Hemangiomas-like: Hyperechoic formation (arrow) with unclear, uneven contours with a dorsal acoustic shadowing (star) |
Figure 4. Ossification: A single hyperechoic structure (arrow) with a strong dorsal acoustic shadowing (star) |
|
Type 5. Metastasis-like: lesions appear as typical structures of liver metastases (for example, colorectal cancer). However, in general, these are hypoechoic solid structures that do not have a typical rim, unlike metastases, and in the center, there is a hyperechoic heterogeneous scar-type pattern (Fig 5, 6).
USG evaluates the size of the liver, the location and segmentation of the parasitic tumor, as well as the extent of involvement of the tubular structures of the liver (46). It also assesses the relationship of the tumor with the inferior vena cava. It describes the ultrasound characteristics of the node (edges, structure, echogenicity, the presence or absence of calcified inclusions and decay cavities) (47). To assess the blood flow, linear blood flow velocity, volumetric blood flow velocity, and resistive indices are determined (48). The criteria for invasion are the uneven contour of the vessel wall, its fragmentary hyper echogenicity, turbulent blood flow, and invasion into the lumen (49). Unlike cystic echinococcosis, there are no cysts in alveolar echinococcosis, so to make diagnosis with an ultrasound is often a difficult task for clinicians and radiologists, especially for those who are unfamiliar with this disease (50).
The use of intravenous contrast in ultrasound examination of the liver can provide important additional data to accurately determine the structure of the parasitic node and assess the local spread of the process (51).
|
|
|
|
Figure 5. Metastasis-like: hyperechoic node (star), without a hypoechoic rim, with a scar in the center |
Figure 6. Metastasis-like: heterogeneous node of reduced density, without the hypoechoic rim, typical of metastases |
It also provides information about the viability and activity of alveococcosis, which helps suggest the prognosis (49).
This method has significant disadvantages, including its dependence on the device and operator, as well as difficulties in detecting small lesions (52). During preoperative preparation, it is essential to complement the obtained data with MRI and multislice CT interpretations to choose the best treatment options (53).
Elastography
Compression elastography and shear wave elastography are used to assess the 'stiffness' and 'elasticity' of pathological liver lesions (54). Alveococcal nodes typically show a high 'stiffness' score when assessed using shear wave elastography (Acoustic Radiation Force Impulse, ARFI). In ARFI VShear (Virtual Touch Quantification Shear Wave) study, the shear wave velocity (SWV) of the pathological node reaches 12.7 m/sec, while the density of the surrounding parenchyma is 2.67 m/sec (or 487 kilopascals (kPa)/22.6 kPa, respectively) (55).
Computed tomography
CT of the abdominal cavity reveals the entire morphological aspect of alveococcal lesions (56). It is one of the primary methods for the objective visualization of parasitic lesions in the liver, allowing for a complete assessment of the anatomical and structural characteristics of the disease (36). It is the best diagnostic method for identifying typical calcified inclusions within lesions, and is especially useful for highly calcified nodules that are difficult to delineate with ultrasound and MRI (57). The use of spiral CT allows determination of the size, number, and location of parasitic foci. It also assesses the degree of invasion into the tubular structures of the liver, which is extremely important for evaluating the extent and resectability of the disease (58). A typical image reveals a tumor-like lesion in the liver with uneven edges and heterogeneous contents (59). These often include calcified inclusions, cysts of various sizes, and areas of necrosis (60).
The generalized primary morphological-tomographic classification of alveolar echinococcosis, known as AEUC (Alveolar Echinococcosis Ulm Classification), developed by T. Graeter, includes five types of foci (61). The AEUC classification, based on computed tomography findings, distinguishes the following five types of alveolar echinococcosis:
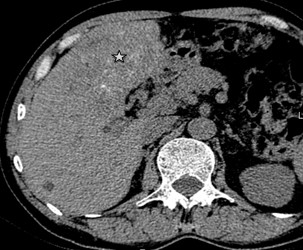
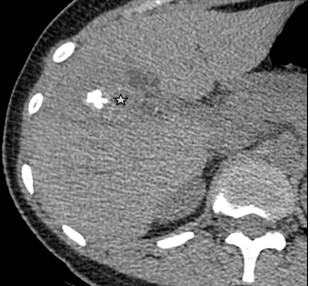
Type 1 – Small cystic/metastasis-like: characterized by irregular, homogeneous nodules with minimal signs of degeneration, without calcification or with central calcification (Fig. 7);
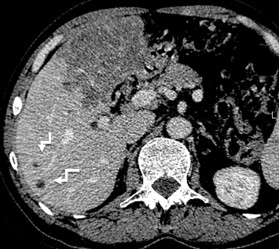
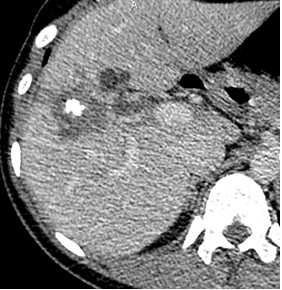
Type 2 – Diffuse-infiltrative: features indistinct, irregular contours, an oval or branching shape, may contain cystic inclusions (fluid necrosis), delicate small focal and linear calcifications (Fig. 8);
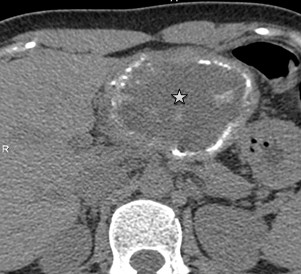
Type 3 – Predominantly circumscribed, tumor-like: has fairly distinct contours, a convex shape, may contain large cystic areas and focal calcifications (Fig. 9);
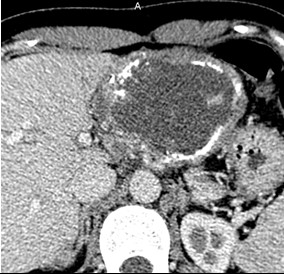
Type 4 – Predominantly cystic: has fairly clear contours, medium to large size, oval or round shape with a dominant cystic component (necrosis), pronounced diffuse calcification (Fig. 10);
Type 5 – Predominantly calcified: the dominant part of the lesion consists of calcified tissue with small areas of necrotic tissue (Fig. 11).
Primary morphology
I Diffuse infiltrating (Fig. 7)
With cystoid portion
Without cystoid portion
II Primarily circumscribed tumor-like
With cystoid portion
Without cystoid portion (Fig. 8)
III (a) Primarily cystoid - intermediate (approximately 3-8 cm)
With more solid portions at the edge
Without more solid portions at the edge
(b) Primarily cystoid - widespread (approximately > 8 cm)
With more solid portions at the edge
Without more solid portions at the edge (Fig. 9)
IV Small-cystoid/metastatic (approximately < 3 cm) (Fig. 10)
V Mainly calcified
|
|
|
|
Figure 7. I- Diffuse-infiltrating type Native phase: Lesion with no clear contours (star), heterogeneous structure of low density with cystic inclusions and small focal calcification |
I- Diffuse-infiltrating type Venous phase: Contrast does not accumulate. In the right lobe of the liver there are single thin-walled liquid cysts (arrows) |
|
|
|
|
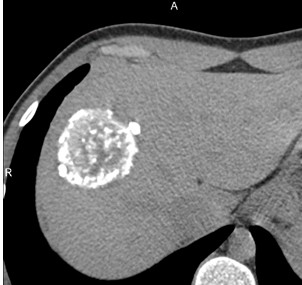
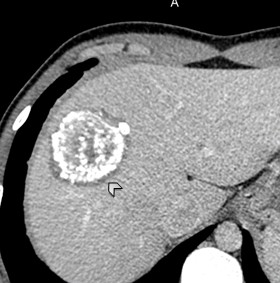
Figure 8. II- Primarily circumscribed tumor-like Native phase: Hyperdense lesion without clear contours (star), irregular oval shape, heterogeneous structure with small calcified inclusions and small cysts. |
II- Primarily circumscribed tumor-like Venous phase: Contours remain unclear, contrast weakly accumulates along the periphery. |
|
|
|
|
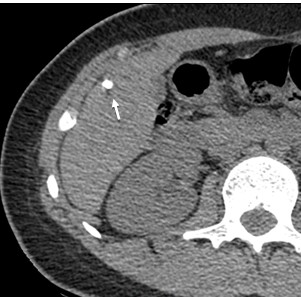
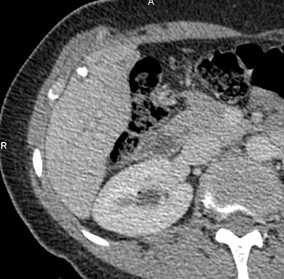
Figure 9. III- Primarily cystoid. Native phase: The formation has a large central cavity (star) with thick walls containing layered and finely focal calcification, small cystic inclusions. |
III- Primarily cystoid. Venous phase: Contrast does not accumulate. |
|
|
|
|
Figure 10. IV- Small-cystoid/metastatic. Native phase: Lesion with central calcification (arrows). |
IV- Small-cystoid/metastatic. Venous phase: Contrast does not accumulate. |
According to Beate Grüner et al., ultrasound classification (EMUC-US) and CT classification of alveococcal nodules of the liver are poorly comparable to each other (62). However, both modalities have superior information value for the diagnosis and differential diagnosis of HAE (63).
Abdominal CT with intravenous bolus contrast is useful for preoperative assessment of vascular invasion, spread of parasitic tumor to adjacent organs and tissues like diaphragm, lungs, stomach, spleen, right kidney, and right adrenal gland (30, 64). CT scans of the chest and brain are recommended before any radical surgery, especially prior to liver transplantation (65).
The 'gold standard' for determining the volume of the postoperative liver remnant (FRL or FRL–V, future remnant liver volume) is volumetry (66).
|
|
|
|
Figure 11. V- Mainly calcified. Native phase: An oval-shaped structure with fairly clear tuberous contours, increased density due to abundant layered and focal calcifications. |
V- Mainly calcified. Venous phase: Around the lesion, there is a perifocal reaction of the hepatic parenchyma in the form of a thin hypovascular layer of low density (arrow head). |
The technique involves determining the total liver volume by calculating the average density values and the number of voxels (67). Then, the liver is segmented according to its vascular architecture, and a virtual 'resection' is performed to calculate the FRL (68). The minimum FRL value is set at 25-30% of the total liver parenchyma volume; however, in cases of morphofunctional disorders (e.g., after chemotherapy), an FRL–V of more than 40% is required. 3D reconstructions and liver segmentation are essential for planning surgical interventions (69).
Post-hepatectomy liver failure is the main complication after liver resection (70). To increase the volume of the future remnant liver, surgical interventions are performed in stages using various regenerative techniques, such as portal vein embolization, associating liver partition and portal vein ligation for staged hepatectomy, and liver venous deprivation (71). Before each step, volumetry is performed to assess FLR hypertrophy and the feasibility of liver resection (72).
CT and positron emission tomography (PET)/CT with labeled fluorodeoxyglucose-18 (18F-FDG) are key methods for diagnosing liver lesions (73). High metabolic activity in alveolar echinococcosis lesions is observed with FDG-PET/CT, especially in areas of microcalcification accumulation (pinnate calcifications or calcifications less than 3 mm) (74). FDG-PET allows indirect identification of parasitic activity and can be used as a tool for further observation (75). PET/CT can detect active lesions even in the absence of clinical symptoms (76). Alveococcal nodes do not have their own circulatory system and are not perfused, so metabolic activity in the marginal zone is associated with inflammatory activity in perifocal tissues (76). It is important to note that a lack of activity on PET does not always indicate the death of the parasite, especially in immunocompromised patients (77). Rather, it reflects the suppression of peri-parasitic inflammation (78). Additionally, at the beginning of treatment with albendazole, inflammatory activity may increase (79).
Magnetic resonance imaging
MRI is a highly sensitive method for differentiating small cystic lesions of hepatic and extrahepatic localization (74). In cases of HAE, MRI reveals a microcystic structure with a solid component, sometimes described as a 'bunch of grapes' or 'honeycomb' (80). The cysts can vary in size—small, medium, or large—and may exhibit a diffuse or uneven distribution at the periphery of the lesion (81). When describing MRI findings of cystic lesions in HAE (Fig. 12, 13), the Kodama classification based on T2-weighted imaging, is commonly used. This classification includes five types (82):
Type 1: Multiple small round cysts without a solid component;
Type 2: Multiple small round cysts with a solid component;
Type 3: A solid component surrounding large and/or irregular cysts, with multiple small round cysts resembling a pseudocyst;
Type 4: A solid component without cysts;
Type 5: A large cyst without a solid component.
|
|
|
|
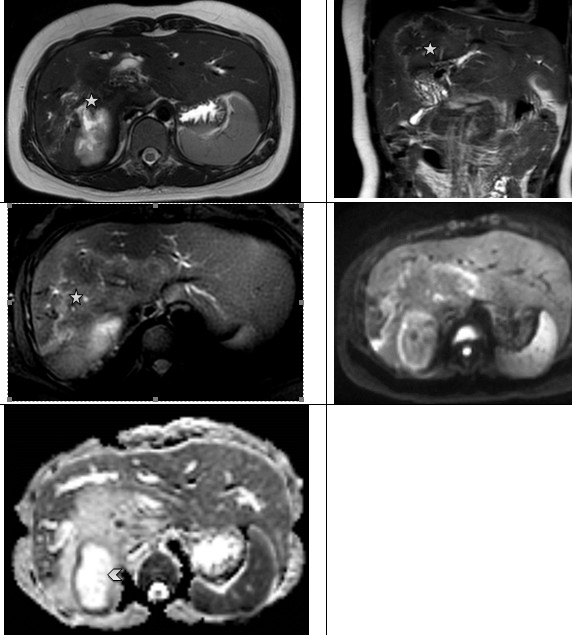
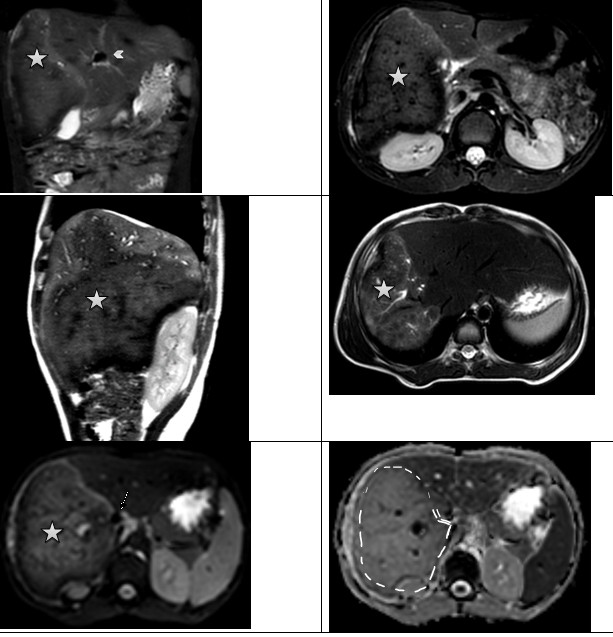
Figure 12. Hepatic alveococcosis. Axial and coronary T2 WI reveal an area of increased heterogenicity in the MR signal (stars), with high diffusion on ADC maps due to the cystic component (arrow head). ADC - apparent diffusion coefficient, MR- magnetic resonance, T2WI - T2-weighted imaging |
|
|
|
|
Figure 13. Hepatic alvecoccosis. In the right lobe of the liver, a large zone of heterogeneous changes in the MR signal is detected with the presence of small cysts along the periphery, with a high-intensity MR signal on T2 WI (star). Invasion into the portal vein (arrow head) and inferior vena cava is noted (arrow). The left lobe of the liver is hypertrophied. On ADC maps, diffusion is higher than in unchanged liver parenchyma (dotted line) ADC - apparent diffusion coefficient, MR- magnetic resonance |
However, over time, it has been recognized that some lesions do not fit into this classification, especially those that
do not contain microcysts. In 2021, a modified Kodama-XUUB (Kodama Xining and Urumqi in China, Ulm in Germany, and Besanßcon in France consortium) classification was proposed to address these limitations. In this new system, type III was divided into subtypes IIIa and IIIb (83).
Tumor-like formations in the liver on MRI images typically show a low signal on T1-weighted imaging, sometimes with an isointense component. On T2-weighted imaging, the signal is usually heterogeneous (84). Areas of necrosis show a high-intensity signal, while some regions exhibit a low signal (85). Diffusion-weighted imaging allows for the differentiation of parasitic nodules from malignant tumors by assessing tissue diffusion and average values on apparent diffusion coefficient maps (86).
To assess the involvement of the biliary tract structures, magnetic resonance cholangiopancreatography (MRCP) is highly effective (87). It provides a complete picture of the relationship between the parasitic process and biliary involvement (88). The informational value of MRCP is comparable to invasive procedures such as percutaneous transhepatic cholangiography and endoscopic retrograde cholangiopancreatography, but it does not require the administration of contrast agents (30). Therefore, MRI should be included in the preoperative evaluation, especially for patients undergoing major liver resection or transplantation (89).
In the study of distant metastases of alveococcosis in the brain, MRI is the preferred method (14). Metastases are usually multiple but can also be singular (90). On T2 and FLAIR-weighted imaging, alveococcal lesions in the brain show a weak signal with multiple small vesicles inside, along with perifocal edema at the periphery (91). On post-contrast T1-weighted images, there is an increased signal from the peripheral parts of the lesion (92).
The final diagnosis of alveococcosis is always based on the results of a morphological study (93). The measured size of the alveococcus cestode, a parasitic larva, usually consists of a conglomerate of multiple microcysts containing excretory capsules, scolexes, and hooks, all bound together by connective tissue (94).
Future directions and challenges
Despite significant advancements in imaging modalities for HAE, several challenges remain in achieving early detection, accurate staging, and optimal treatment planning. Future research should focus on enhancing imaging techniques, improving diagnostic algorithms in endemic regions, and integrating artificial intelligence (AI) into image analysis. Contrast-enhanced ultrasound has shown promise in differentiating HAE lesions from malignant tumors by assessing microvascularization patterns in real time. It is a cost-effective and widely available tool that can be particularly useful in low-resource endemic regions. PET/CT, particularly with 18F-FDG, can provide valuable metabolic information about parasitic viability and treatment response. This modality may help in assessing occult metastases and residual disease activity, potentially influencing long-term management strategies.
Improving Diagnostic Algorithms in Endemic Regions
Many endemic areas lack access to advanced imaging modalities like CT and MRI, leading to delays in diagnosis and treatment. To address this issue:
•Efforts should be made to develop standardized ultrasound-based diagnostic protocols, enabling primary healthcare providers to detect HAE at an early stage.
•Portable ultrasound devices combined with telemedicine solutions could improve access to diagnostic services in rural areas.
•Enhancing medical training programs for radiologists and general practitioners in endemic regions will facilitate earlier detection and referral for specialized imaging.
The Role of Machine Learning in HAE Imaging
The integration of AI and machine learning (ML) into radiology is expected to revolutionize the diagnosis of HAE. AI-driven image analysis algorithms could help automate the detection of HAE lesions on ultrasound, CT, and MRI scans, improving diagnostic accuracy and reducing human error. Deep learning models trained on large datasets of echinococcosis cases could assist in differentiating HAE from malignant liver tumors and other hepatic lesions. AI applications in radiomics and texture analysis may provide insights into disease progression, treatment response, and prognosis, enabling personalized patient management (95).
Conclusion
The global epidemiological situation regarding the prevalence of human alveococcosis has remained consistently tense over the past decades. HAE has a distinct radiological appearance, but the parasitic process is often mistakenly diagnosed as a malignant liver tumor. Among diagnostic modalities, ultrasound remains the primary method, continuously improving with the introduction of elastography and contrast-enhanced techniques. CT with bolus contrast is essential for all patients with hepatic alveococcosis, as it provides valuable diagnostic information. Implementing CT volumetry is highly recommended for accurately determining the residual volume of the liver parenchyma. MRI is an excellent tool for evaluating vascular and biliary tract involvement. Currently, MR cholangiopancreatography has largely replaced percutaneous cholangiography in assessing the relationship between HAE lesions and the biliary tree. Preoperative preparation should also include CT of the lungs and brain. To assess the effectiveness of conservative therapy, FDG-PET can be used to determine the metabolic activity of the parasite. The final diagnosis of HAE is always established based on the results of a morphological study.
The larva of the alveococcus typically consists of a conglomerate of multiple microcysts containing excretory capsules, scolexes, and hooks, all bound together by connective tissue.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: l.B., A.K., K.I.,P.K.C., B.B., S.A.,
T.U., and K.R. equally contributed to the study, preparation of manuscript, and fulfilled authorship criteria
Acknowledgement and funding: None to declare
Statement on A.I.-assisted technologies use: Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Do not apply
References
| 1. Guidelines for treatment of cystic and alveolar echinococcosis in humans. WHO Informal Working Group on Echinococcosis. Bull World Health Organ 1996; 74: 231-42. | ||||
| 2.Meinel TR, Gottstein B, Geib V, Keel MJ, Biral R, Mohaupt M , Jet al. Vertebral alveolar echinococcosis-a case report, systematic analysis, and review of the literature. Lancet Infect Dis 2018; 18: e87-e98. doi: 10.1016/S1473-309917.30335-3 https://doi.org/10.1016/S1473-3099(17)30335-3 PMid:28807628 |
||||
| 3. Vuitton DA, Wang Q, Zhou HX, Raoul F, Knapp J, Bresson-Hadni S, et al. A historical view of alveolar echinococcosis, 160 years after the discovery of the first case in humans: part 1. What have we learnt on the distribution of the disease and on its parasitic agent? Chin Med J Engl 2011; 12418: 2943-53 | ||||
| 4. Spotin A, Boufana B, Ahmadpour E, Casulli A, Mahami-Oskouei M, Soheila Rouhani S, et al. Assessment of the global pattern of genetic diversity in Echinococcus multilocularis inferred by mitochondrial DNA sequences. Vet Parasitol 2018; 262: 30-41. doi: 10.1016/j.vetpar.2018.09.013 https://doi.org/10.1016/j.vetpar.2018.09.013 PMid:30389009 |
||||
| 5.Ali R, Khan S, Khan M, Adnan M, Ali I, Khan TA, Haleem S, et al. A systematic review of medicinal plants used against Echinococcus granulosus. PLoS ONE 2020; 15: doi: 10.1371/journal.pone.0240456 https://doi.org/10.1371/journal.pone.0240456 PMid:33048959 PMCid:PMC7553295 |
||||
| 6.Torgerson PR, Devleesschauwer B, Praet N, Speybroeck N, Willingham AL, Kasuga F, et al. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: A Data Synthesis. PLoS Med 2015 https://doi.org/10.1371/journal.pmed.1001920 PMid:26633705 PMCid:PMC4668834 |
||||
| 12: 2015. doi: 10.1371/journal.pmed.1001920 https://doi.org/10.1371/journal.pmed.1001920 PMid:26633705 PMCid:PMC4668834 |
||||
| 7.Kholin AV, Amanbaeva GT, Kakishev UK. Diagnostic capacity of X-RAY computed tomography and mri in recognition of hepatic alveococcossis and echinococcosis. Vest KRSU 2015; 5: 164-9. 2015. | ||||
| 8.Massolo A, Santa MA, Musiani M, Ruckstuhl KE. A review on invasions by parasites with complex life cycles: the European strain of Echinococcus multilocularis in North America as a model. Parasitol 2021; doi: 10.1017/S0031182021001426 https://doi.org/10.1017/S0031182021001426 PMid:35060461 PMCid:PMC8564803 |
||||
| 9.Dezsényi B, Dubóczki Z, Strausz T, Csulak E, Czoma V, Káposztáset Z, al. Emerging human alveolar echinococcosis in Hungary 2003-2018.: a retrospective case series analysis from a multi-centre study', BMC Infect Dis 2021; 21: doi: 10.1186/s12879-021-05859-5. https://doi.org/10.1186/s12879-021-05859-5 PMid:33568075 PMCid:PMC7877032 |
||||
| 10.Chen J, Wei L, Deng CM, Xiong J, Chen SM, Lu D, et al. A liver CT based nomogram to preoperatively predict lung metastasis secondary to hepatic alveolar echinococcosis. Eur J Radiol 2025; 183: 111865. doi: 10.1016/j.ejrad.2024.111865 https://doi.org/10.1016/j.ejrad.2024.111865 PMid:39644597 |
||||
| 11.Zhou Y, Feng P, Tian F, Fong H, Yang H, Zhu H. A CT-based radiomics model for predicting lymph node metastasis in hepatic alveolar echinococcosis patients to support lymph node dissection. Eur J Med Res 2024; 29: 409. doi: 10.1186/s40001-024-01999-x https://doi.org/10.1186/s40001-024-01999-x PMid:39113113 PMCid:PMC11304587 |
||||
| 12. Yesilyurt M, Polat G. A rare cause of alveolar echinococcal metastasis. Rev Soc Bras Med Trop 2023; 56: doi: 10.1590/0037-8682-0435-2022 https://doi.org/10.1590/0037-8682-0435-2022 PMid:36820660 PMCid:PMC9957113 |
||||
| 13. Aydin F, Yalcin A, Karaman A, Sade R, Ozturk G, Alper F. Diagnostic and management perspectives in alveolar echinococcosis: Review of literature. Eurasian J Med 2023; 54: S10-5. doi: 10.5152/eurasianjmed.2022.22308 https://doi.org/10.5152/eurasianjmed.2022.22308 PMid:36655439 PMCid:PMC11163348 |
||||
| 14.Trofimova TN, Amanbaeva GT. Cerebral hydatid lesions. Diagn Radiol Radiother 2016; 2: 37-46. doi: 10.22328/2079-5343-2016-2-37-46 https://doi.org/10.22328/2079-5343-2016-2-37-46 |
||||
| 15.Joliat GR, Joliat GR, Sebastiao N Martins-Filho SN, Haefliger S, Demartines N, et al. Programmed death-ligand1 is a determinant of recurrence in alveolar echinococcosis. Int J Infect Dis 2023; 129: 285-8. doi: 10.1016/j.ijid.2023.01.043. https://doi.org/10.1016/j.ijid.2023.01.043 PMid:36775187 |
||||
| 16.Hu Q, Chen S, Fan Y, Lu Q, Deng M, Fan H. Kidney invasion occurred 2 years following liver transplantation for hepatic alveolar echinococcosis: a case report. BMC Infect Dis 2023; 23: 785. doi: 10.1186/s12879-023-08788-7 https://doi.org/10.1186/s12879-023-08788-7 PMid:37950231 PMCid:PMC10638689 |
||||
| 17. Bebezov BK, Bebezov KhS, Umetaliev TM, Mamashev ND, Surov EA, Ryspekov BZ, et al., 'advanced liver resections in alveococcosis. Vest KRSU 2022; 8: doi: 10.36979/1694-500X-2022-22-1-23-29 https://doi.org/10.36979/1694-500X-2022-22-1-23-29 |
||||
| 18.Nahorski WL, Knap JP, Pawlowski ZS, Krawczyk M, Polanski J, Stefaniak J, et al. Human alveolar echinococcosis in Poland: 1990-2011. PLoS Negl Trop Dis 2013; 7: doi: 10.1371/journal.pntd.0001986 https://doi.org/10.1371/journal.pntd.0001986 PMid:23301116 PMCid:PMC3536814 |
||||
| 19. Zagainov VE, Porshennikov IA, Kiselev NM, Naydenov EV, Pavlik VN, Voskanyan SE. New classification of alveolar echinococcosis of the liver as a base of new surgical strategy. A multicenter study. Ann HPB Surg 2021; 25: 20-32. doi: 10.16931/1995-5464.2020420-32 https://doi.org/10.16931/1995-5464.2020420-32 |
||||
| 20.Pielok P, Karczewski M, Cierach W, Zmora P, Lenartowicz E, Stefaniak J. Portal hypertension as a result of the incomplete surgically treated advanced alveolar echinococcosis: A case description. BMC Gastroenterol 2020; 20: doi: 10.1186/s12876-020-01320-0. https://doi.org/10.1186/s12876-020-01320-0 PMid:32503447 PMCid:PMC7275433 |
||||
| 21.Omorov RA, Aitbaev SA, Kanietov AK, Abdiev AA. Results of surgical treatment of patients with liver alveolar echinococcosis. Ann HPB Surg 2018; 23: 74-9. doi: 10.16931/1995-5464.2018-1-74-79 https://doi.org/10.16931/1995-5464.2018-1-74-79 |
||||
| 22.Botiraliev AS, Stepanova YV, Vishnevskii VA, Jao C. Prediction of biliary complications after liver resection. Vestnik SURG 2021; 14: 226-8. doi: 10.52090/2542-1646_2021_9_1_226 | ||||
| 23. Kantarci M, Bayraktutan U, Karabulut N, Aydinli B, Ogul H, Yuce I, et al. Alveolar echinococcosis: Spectrum of findings at cross-sectional imaging. Radiographics 2012 https://doi.org/10.1148/rg.327125708 PMid:23150858 |
||||
| 32: 2053-70. 2012, doi: 10.1148/rg.327125708 https://doi.org/10.1148/rg.327125708 PMid:23150858 |
||||
| 24.Abdiev AA. Treatment of pleuropulmonary complications after liver resections for alveolar echinococcosis. Euras J Health 2021; . 3: 71-3. | ||||
| 25. Castillo S, Manterola C, Grande L, Rojas C. Infected hepatic echinococcosis. Clinical, therapeutic, and prognostic aspects. A systematic review. Ann Hepatol. 2021; 22: 100237. doi: 10.1016/j.aohep.2020.07.009 https://doi.org/10.1016/j.aohep.2020.07.009 PMid:32835861 |
||||
| 26.Craig PS, Giraudoux P, Wang ZH, Wang Q. Echinococcosis transmission on the Tibetan Plateau. Adv Parasitol 2019; 104: 165-246. doi: 10.1016/bs.apar.2019.03.001 https://doi.org/10.1016/bs.apar.2019.03.001 PMid:31030769 |
||||
| 27.Haberman DM, et al. Role of CT in two-stage liver surgery. RadioGraphics 2022; 42: 106-24. doi: 10.1148/rg.210067. https://doi.org/10.1148/rg.210067 PMid:34990325 |
||||
| 28.Jiang T, et al. Collateral circulation caused by end-stage hepatic alveolar echinococcosis. BMC Infect Dis 2023; 23: 322. doi: 10.1186/s12879-022-07970-7 https://doi.org/10.1186/s12879-022-07970-7 PMid:37189056 PMCid:PMC10184362 |
||||
| 29. Peng Y, et al. CT-derived extracellular volume and liver volumetry can predict posthepatectomy liver failure in hepatocellular carcinoma. Insights Imag 2023; 14: 145. doi: 10.1186/s13244-023-01496-5 https://doi.org/10.1186/s13244-023-01496-5 PMid:37697217 PMCid:PMC10495294 |
||||
| 30. Bulakçi M, Demir A, Öztürk HE, Kalkan M, Şahin E, Kızılgöz F. Multimodality imaging in diagnosis and management of alveolar echinococcosis: an update. Diagn Interv Radiol 2016; 22: 247-56. doi: 10.5152/dir.2015.15456 https://doi.org/10.5152/dir.2015.15456 PMid:27082120 PMCid:PMC4859741 |
||||
| 31.Zerial M, Lorenzin D, Risaliti A, Zuiani C, R. Girometti R. Abdominal cross-sectional imaging of the associating liver partition and portal vein ligation for staged hepatectomy procedure. World J Hepatol 2017; 9: 733-45. doi: 10.4254/wjh.v9.i16.733 https://doi.org/10.4254/wjh.v9.i16.733 PMid:28652892 PMCid:PMC5468342 |
||||
| 32. Yang X, et al. Risk factors and a simple model for predicting bile leakage after radical hepatectomy in patients with hepatic alveolar echinococcosis. Med US 2017; 96: doi: 10.1097/MD.0000000000008774 https://doi.org/10.1097/MD.0000000000008774 PMid:29145333 PMCid:PMC5704878 |
||||
| 34. Yurkovskaya A, Marinova L, Chzhao A, Vladimirovich AC. Alveococcosis of the liver: clinic, diagnosis, treatment literature review. XXX 2022; 47: doi: 10.52090/2542-1646_2021_9_1_47 | ||||
| 35. Kern P, et al. WHO classification of alveolar echinococcosis: Principles and application. Parasitol Int 2006; doi: 10.1016/j.parint.2005.11.041 https://doi.org/10.1016/j.parint.2005.11.041 PMid:16343985 |
||||
| 36. Chouhan MD, Wiley E, Chiodini PL, Amin Z. Hepatic alveolar hydatid disease Echinococcus multilocularis, a mimic of liver malignancy: a review for the radiologist in non-endemic areas. Clin Radiol 2019;. 74: 247-56. doi: 10.1016/j.crad.2019.01.007 https://doi.org/10.1016/j.crad.2019.01.007 PMid:30755313 |
||||
| 37. Aydin S, Irgul B, Memis KB, Kizilgoz V, Kantarci M. Characteristics of the imaging diagnosis of alveolar echinococcosis. World J. Gastrointest Surg 2024; 16: 2748-54. doi: 10.4240/wjgs.v16.i9.2748 https://doi.org/10.4240/wjgs.v16.i9.2748 PMid:39351560 PMCid:PMC11438814 |
||||
| 38. Strohäker J, Nadalin S. Diagnostics and management of intra-abdominal Echinococcus manifestation. Chirurg 2019; 90: 823-32. doi: 10.1007/s00104-019-1001-6 https://doi.org/10.1007/s00104-019-1001-6 PMid:31312861 |
||||
| 39. Liu W, et al. Innovation in hepatic alveolar echinococcosis imaging: best use of old tools, and necessary evaluation of new ones. Parasite 2014; doi: 10.1051/parasite/2014072 https://doi.org/10.1051/parasite/2014072 PMid:25531446 PMCid:PMC4273719 |
||||
| 40. Gao GH, et al. Field evaluation of an immunochromatographic test for diagnosis of cystic and alveolar echinococcosis. Parasit Vectors 2018; 11: 311. doi: 10.1186/s13071-018-2896-3 https://doi.org/10.1186/s13071-018-2896-3 PMid:29792228 PMCid:PMC5966859 |
||||
| 41. Sulima M, et al. Ultrasound images in hepatic alveolar echinococcosis and clinical stage of the disease. Adv Med Sci 2019; 64: 324-30. doi: 10.1016/j.advms.2019.04.002 https://doi.org/10.1016/j.advms.2019.04.002 PMid:31003201 |
||||
| 42. Ashivkina OI. Hepatic alveolar echinococcosis: the possibilities of ultrasonography. Planning surgical operation. Med Vis 2017; 3: 32-48. doi: 10.24835/1607-0763-2017-3-32-43 https://doi.org/10.24835/1607-0763-2017-3-32-43 |
||||
| 43.Schweizer M, Schmidberger J, Schlingeloff P, Kratzer W. Contrast-enhanced ultrasound CEUS. in patients with metastasis-like hepatic alveolar echinococcosis: a cohort study. J Ultrasound 2022; doi: 10.1007/s40477-022-00688-x https://doi.org/10.1007/s40477-022-00688-x PMid:35597873 PMCid:PMC10063733 |
||||
| 44.Dietrich CF, et al. Cystic and alveolar echinococcosis of the hepatobiliary tract - the role of new imaging techniques for improved diagnosis. Med Ultrason 2020; 22: 75-84. doi: 10.11152/mu-2421 https://doi.org/10.11152/mu-2421 PMid:32096792 |
||||
| 45.Kratzer W, et al. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J. Gastroenterol 2015; 21: 12392-402. doi: 10.3748/wjg.v21.i43.12392 https://doi.org/10.3748/wjg.v21.i43.12392 PMid:26604646 PMCid:PMC4649122 |
||||
| 46.Dong CH, Lu Q, Wang WP, Ji ZB, Wang X. Contrast-enhanced ultrasound features of hepatic reactive lymphoid hyperplasia: correlation with histopathologic findings. J Ultrasound Med 2019; 38: 2379-88. https://doi.org/10.1002/jum.14934 PMid:30666662 |
||||
| 47.Wang YX, Liu W, Sun ZY, Wu L, Xie XK, Liu B. Analysis of ultrasonographic characteristics of early hepatic alveolar echinococcosis. Front Surg 2022; 9: 918138. doi: 10.3389/fsurg.2022.918138 https://doi.org/10.3389/fsurg.2022.918138 PMid:35865038 PMCid:PMC9294286 |
||||
| 48.Wa ZC, et al. Differential diagnosis between hepatic alveolar echinococcosis and intrahepatic cholangiocarcinoma with conventional ultrasound and contrast-enhanced ultrasound. BMC Med Imaging 2020; 20: 101. doi: 10.1186/s12880-020-00499-8 https://doi.org/10.1186/s12880-020-00499-8 PMid:32854653 PMCid:PMC7453544 |
||||
| 49.Stepanova YuA, Ashivkina OI, Ionkin DA, Glotov AV, Vishnevsky VA. Liver alveococcosis: ultrasound and morphological comparisons. Mol Meditsina Mol Med 2020; 18: doi: 10.29296/24999490-2020-01-09 https://doi.org/10.29296/24999490-2020-01-09 |
||||
| 50.Peters L, Burkert S, Grüner B. Parasites of the liver - epidemiology, diagnosis and clinical management in the European context. 2021, doi: 10.1016/j.jhep. https://doi.org/10.1016/j.jhep.2021.02.015 PMid:33636243 |
||||
| 51.Zhang X, et al. Can contrast-enhanced ultrasound differentiate the type of hepatic echinococcosis: cystic echinococcosis or alveolar echinococcosis? Parasit Vectors 2023; 16: 131. doi: 10.1186/s13071-023-05731-2 https://doi.org/10.1186/s13071-023-05731-2 PMid:37069610 PMCid:PMC10111660 |
||||
| 52. Tao Y, et al. Pictorial review of hepatic echinococcosis: Ultrasound imaging and differential diagnosis. World J Gastroenterol 2024; 30: 4115-31. doi: 10.3748/wjg.v30.i37.4115 https://doi.org/10.3748/wjg.v30.i37.4115 PMid:39474399 PMCid:PMC11514533 |
||||
| 53 Voskanyan E, Bashkov AN, Karmazanovsky GG, Naydenov EV, Ionova EA. Planning principles for radical surgical intervention for liver alveococcosis based on computed and magnetic resonance imaging. Ann HPB Surg 2020; 25: doi: 10.16931/1995-5464.20202100-112 https://doi.org/10.16931/1995-5464.20202100-112 |
||||
| 54.Ghiuchici AM, Danila M. The place of elastography for liver tumors assessment. In: Stoian D, Popescu A. (editors). Elastography. Rijeka: IntechOpen; 2022. doi: 10.5772/intechopen.103777 https://doi.org/10.5772/intechopen.103777 |
||||
| 55.Zagaynov VE, Kiselev NM, Belskiy VA, Rykhtik PI, Bobrov NV. "Big trifles" of advanced liver resections for alveococcosis. Ann HPB Surg 2019; 23: 33-44. doi: 10.16931/1995-5464.2018433-44 https://doi.org/10.16931/1995-5464.2018433-44 |
||||
| 56. Eroglu A, Ogul H, Aydin Y. CT imaging findings of pulmonary alveolar echinococcosis. Curr Med Imaging 2023; 19: 97-102. doi: 10.2174/1573405618666220128160440 https://doi.org/10.2174/1573405618666220128160440 PMid:35088676 |
||||
| 57.Bogdanov A. X-ray computed tomography and magnetic resonance image alveolar disease of liver diagnostic. Vest KRSU 2017; 3: 119-22. | ||||
| 58. Voskanyan SE, Bashkov AN, Karmazanovsky GG, Naydenov EV, Ionova EA. Planning principles for radical surgical intervention for liver alveococcosis based on computed and magnetic resonance imaging. Ann HPB Surg 2020; 25: 100-12. doi: 10.16931/1995-5464.20202100-112 https://doi.org/10.16931/1995-5464.20202100-112 |
||||
| 59. Chen J et al. Triphase contrast-enhanced CT to evaluate indications for autologous liver transplantation in patients with end-stage hepatic alveolar echinococcosis. Sci Rep 2021; 11: doi: 10.1038/s41598-021-01586-8. https://doi.org/10.1038/s41598-021-01586-8 PMid:34764382 PMCid:PMC8586367 |
||||
| 60.Graeter T, et al. Hepatic alveolar echinococcosis: Comparative computed tomography study between two Chinese and two European centres. Food Waterborne Parasitol 2020; 19: e00082. doi: 10.1016/j.fawpar.2020.e00082 https://doi.org/10.1016/j.fawpar.2020.e00082 PMid:32435708 PMCid:PMC7232088 |
||||
| 61.Graeter T, Schmidberger J. Stage-oriented CT classification and intermodal evolution model in hepatic alveolar echinococcosis. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr 2022; 194: 532-44. doi: 10.1055/a-1710-3669. https://doi.org/10.1055/a-1710-3669 PMid:35081647 PMCid:PMC9133419 |
||||
| 62.Grüner B, Schmidberger j, Drews O, Kratzer W, Gräter T. Imaging in alveolar echinococcosis AE: Comparison of Echinococcus multilocularis classification for computed-tomography EMUC-CT and ultrasonography EMUC-US. Radiol Infect Dis 2017; 4: 70-7. doi: 10.1016/j.jrid.2017.05.001.60 https://doi.org/10.1016/j.jrid.2017.05.001 |
||||
| 63. Kantarci M, Aydin S, Eren S, Ogul H, AkhanO. Imaging aspects of hepatic alveolar echinococcosis: retrospective findings of a surgical center in Turkey. Pathogens 2022; 11: doi: 10.3390/pathogens11020276 https://doi.org/10.3390/pathogens11020276 PMid:35215218 PMCid:PMC8877742 |
||||
| 64. Bashkov AN, Voskanyan SE, Karmazanovsky GG, Naydenov EV, Ionova EA. Planning of the autotransplantation of the liver to the patients with advanced alveococcosis based on the multidetector computed tomography. Med Vis 2017; 4: 123-31. doi: 10.24835/1607-0763-2017-4-123-131 https://doi.org/10.24835/1607-0763-2017-4-123-131 |
||||
| 65.Kiselev NM, Zagainov VE. The results of surgical treatment in patients with liver alveococcosis in a hepato-pancreato-biliary center: A 10-years' experience. Alm Clin Med 2018; 46: 609-17. doi: 10.18786/2072-0505-2018-46-6-609-617 https://doi.org/10.18786/20720505-2018-46-6-609-617 |
||||
| 66.Voskanyan SE, Bashkov AN, Karmazanovsky GG, Naydenov EV, Ionova EV. ALPPS in overcoming small remnant liver volume in alveolar echinococcosis. Ann. HPB Surg 2019; 23: 21-32. doi: 10.16931/1995-5464.2018421-32 https://doi.org/10.16931/1995-5464.2018421-32 |
||||
| 67. Q. Guo et al., "Application of Hepatic Lobe Hyperplasia Techniques in the Treatment of Advanced Hepatic Alveolar Echinococcosis: A Single-Centre Experience," BMC Surg., vol. 22, no. 1, p. 415, Dec. 2022, doi: 10.1186/s12893-022-01864-w. https://doi.org/10.1186/s12893-022-01864-w PMid:36474286 PMCid:PMC9724394 |
||||
| 68.Tsukano Y, Sugita M, Hirata N, Yamamoto T. Future liver remnant volume is associated with postoperative fentanyl consumption following open donor hepatectomy: A retrospective multivariate analysis. J Anesth 2022; 36: 731-39. doi: 10.1007/s00540-022-03110-2 https://doi.org/10.1007/s00540-022-03110-2 PMid:36190573 |
||||
| 69. Gavriilidis P, Marangon G, Ahmad J, Azoulay D. Simultaneous portal and hepatic vein embolization is better than portal embolization or alpps for hypertrophy of future liver remnant before major hepatectomy: A systematic review and network meta-analysis. Hepatobiliary Pancreat Dis Int 2022; doi: 10.1016/j.hbpd.2022.08.013 https://doi.org/10.1016/j.hbpd.2022.08.013 PMid:36100542 |
||||
| 70.Fernandez H, Nadalin S, Testa G. Optimizing future remnant liver prior to major hepatectomies: increasing volume while decreasing morbidity and mortality. Hepatobiliary Surg Nutr 2020; 9: 215-8. doi: 10.21037/hbsn.2019.10.24 https://doi.org/10.21037/hbsn.2019.10.24 PMid:32355683 PMCid:PMC7188535 |
||||
| 71. Balci D, Sakamoto Y, Li J, Di Benedetto F, Kirimker EO, Petrowsky H. Associating liver partition and portal vein ligation for staged hepatectomy (alpps) procedure for cholangiocarcinoma. Int J Surg 2020; 82S: 97-102. doi: 10.1016/j.ijsu.2020.06.045 https://doi.org/10.1016/j.ijsu.2020.06.045 PMid:32645441 |
||||
| 72. Yi F, Zhang W, Feng L. Efficacy and safety of different options for liver regeneration of future liver remnant in patients with liver malignancies: A systematic review and network meta-analysis. World J Surg Oncol 2022; 20: doi: 10.1186/s12957-022-02867-w https://doi.org/10.1186/s12957-022-02867-w PMid:36527081 PMCid:PMC9756618 |
||||
| 73. Orita T, Sato T, Nakamura Y, Yamaguchi H, Kaneko Y, . Kawabata Y, et al. FDG-PET/CT: Novel method for viability assessment of livers perfused ex vivo. Nucl Med Commun 2021; 42: 826-32. doi: 10.1097/MNM.0000000000001399 https://doi.org/10.1097/MNM.0000000000001399 PMid:33741853 |
||||
| 74. Graeter T, Rausch AK, Müller A, Schmidt TE, Nagel DH, Beyer C, et al. Hepatic alveolar echinococcosis: correlation between computed tomography morphology and inflammatory activity in positron emission tomography. Sci Rep 2020; 10: 11808. doi: 10.1038/s41598-020-68624-9 https://doi.org/10.1038/s41598-020-68624-9 PMid:32678174 PMCid:PMC7366930 |
||||
| 75. Husmann L, Haenni JGW, Peters DTH, Christou MPR. PET/CT helps to determine treatment duration in patients with resected as well as inoperable alveolar echinococcosis. Parasitol Int 2021; 83: 102356. doi: 10.1016/j.parint.2021.102356 https://doi.org/10.1016/j.parint.2021.102356 PMid:33872794 |
||||
| 76. Husmann L, Müller MO, Weber PD. Follow-up PET/CT of alveolar echinococcosis: comparison of metabolic activity and immunodiagnostic testing. PloS One 2022; 17: e0270695. doi: 10.1371/journal.pone.0270695 https://doi.org/10.1371/journal.pone.0270695 PMid:35767557 PMCid:PMC9242476 |
||||
| 77.Gottstein B, Kestler FGP, Reinhardt RRW. Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Food Waterborne Parasitol 2019; 16: e00055. doi: 10.1016/j.fawpar.2019.e00055 https://doi.org/10.1016/j.fawpar.2019.e00055 PMid:32095626 PMCid:PMC7034017 |
||||
| 78.Husmann L, Meier AM, Schimmel SF, Nesser RMN. Prediction of benzimidazole therapy duration with PET/CT in Inoperable patients with alveolar echinococcosis. Sci Rep 2022; 12: 11392. doi: 10.1038/s41598-022-15641-5 https://doi.org/10.1038/s41598-022-15641-5 PMid:35794149 PMCid:PMC9259695 |
||||
| 79. Ricken FJ, Schoenberg HR, John CWS. Albendazole increases the inflammatory response and the amount of Em2-positive small particles of echinococcus multilocularis spem in human hepatic alveolar echinococcosis lesions. PLoS Negl Trop Dis 2017; 11: doi: 10.1371/journal.pntd.0005636 https://doi.org/10.1371/journal.pntd.0005636 PMid:28542546 PMCid:PMC5462468 |
||||
| 80.Aoki T, Hagiwara M, Yabuki H, Ito A. Unique MRI findings for differentiation of an early stage of hepatic alveolar echinococcosis. BMJ Case Rep 2015; 2015: bcr2014208123. doi: 10.1136/bcr-2014-208123 https://doi.org/10.1136/bcr-2014-208123 PMid:25697300 PMCid:PMC4336886 |
||||
| 81.Calame P, Kohler DRMW, Lichtenstein LH. Role of the radiologist in the diagnosis and management of the two forms of hepatic echinococcosis. Insights Imaging 2022; 13: 68. doi: 10.1186/s13244-022-01190-y https://doi.org/10.1186/s13244-022-01190-y PMid:35394226 PMCid:PMC8994011 |
||||
| 82.Kodama Y, Watanabe T, Nakanishi H, Fujimoto M. Alveolar echinococcosis: MR findings in the liver. Radiology 2003; 228: 172-7. doi: 10.1148/radiol.2281020323 https://doi.org/10.1148/radiol.2281020323 PMid:12750459 |
||||
| 83.Brumpt E, Valente MHZ, Phan SK. Kodama-XUUB: An informative classification for alveolar echinococcosis hepatic lesions on magnetic resonance imaging. Parasite 2021; 28: 66. doi: 10.1051/parasite/2021062 https://doi.org/10.1051/parasite/2021062 PMid:34569927 PMCid:PMC8475500 |
||||
| 84.Parry AH, Wani AH, Feroz I. The Spectrum of multimodality imaging findings in hepatic alveolar echinococcosis and the potential role of diffusion-weighted imaging in its characterization. Pol J Radiol 2020; 85: 613-23. doi: 10.5114/pjr.2020.101015 https://doi.org/10.5114/pjr.2020.101015 PMid:33376563 PMCid:PMC7757515 |
||||
| 85.Ren B, Wu T, Zhang QS, Luo YY. Hepatic alveolar echinococcosis: Predictive biological activity based on radiomics of MRI. BioMed Res. Int., vol. 2021, no. 1, p. 6681092, Jan. 2021, doi: 10.1155/2021/6681092. https://doi.org/10.1155/2021/6681092 PMid:33997041 PMCid:PMC8108638 |
||||
| 86.Kantarci M, Pirimoglu B. Diffusion-weighted MR imaging findings in a growing problem: hepatic alveolar echinococcosis. Eur J Radiol 2014; 83: 1991-2. doi: 10.1016/j.ejrad.2014.07.013 https://doi.org/10.1016/j.ejrad.2014.07.013 PMid:25112672 |
||||
| 87.Al-Asbah Hi, Heffernan MM, Miller SBA. Intra-biliary hydatid cyst rupture: A rare case report with superinfection. Clin. Case Rep 2024L 12: e8581. doi: 10.1002/ccr3.8581 https://doi.org/10.1002/ccr3.8581 PMid:38500781 PMCid:PMC10944800 |
||||
| 88.Zhang N, Chen Y, Tang L, Zhou D, Hou T. Diagnosis of bronchobiliary fistula by bilirubin crystallization in the alveolar lavage fluid: case reports and literature review. Ann Palliat Med 2021; 10: 7121-5. doi: 10.21037/apm-21-1040 https://doi.org/10.21037/apm-21-1040 PMid:34237991 |
||||
| 89. Yuan J, Xu ZR, Zhang LK. Single-center experience of ex vivo liver resection and autotransplantation for complex hepatic alveolar echinoccosis. front Surg 2023; 10: 1089788. doi: 10.3389/fsurg.2023.1089788.95 https://doi.org/10.3389/fsurg.2023.1089788 PMid:36874451 PMCid:PMC9975350 |
||||
| 90. Ju H, Liu C. Cerebral alveolar echinococcosis. N Engl J Med 2023; 388: 453. doi: 10.1056/NEJMicm2202196 https://doi.org/10.1056/NEJMicm2202196 PMid:36724380 |
||||
| 91. Yimit Y, Liu ME, Zhang Y, Zhuang Y, Zeng Y, Zhang MY. Differentiation between cerebral alveolar echinococcosis and brain metastases with radiomics combined machine learning approach. Eur J Med Res 2023; 28: 577. doi: 10.1186/s40001-023-01550-4 https://doi.org/10.1186/s40001-023-01550-4 PMid:38071384 PMCid:PMC10709961 |
||||
| 92. Li S, Zhang B, Zhang T, Zhang X, Liu H. Clinical Features, Radiological Characteristics, and Outcomes of Patients With Intracranial Alveolar Echinococcosis: A Case Series From Tibetan Areas of Sichuan Province, China. Front Neurol 2021; 11: doi: 10.3389/fneur.2020.537565 https://doi.org/10.3389/fneur.2020.537565 PMid:33519658 PMCid:PMC7843382 |
||||
| 93. Alvi MA, Iqbal MA, Ali T, Hussain S. Past and present of diagnosis of echinococcosis: A review 1999-2021. Acta Trop 2023; 243: 106925. doi: 10.1016/j.actatropica.2023.106925 https://doi.org/10.1016/j.actatropica.2023.106925 PMid:37080264 |
||||
| 94. Gottstein B, Romig KL, Vetter WH. Echinococcus metacestode: in search of viability markers. 2014, doi: 10.1051/parasite/2014063 https://doi.org/10.1051/parasite/2014063 PMid:25429386 PMCid:PMC4245873 |
||||
| 95. Wang Z, Guo H, Xu L, Liu H, Zhang X, Chen Z, ET AL. Detection and subtyping of hepatic echinococcosis from plain CT images with deep learning: a retrospective, multicentre study. Lancet Digit Health 2023; 5:. e754-62. doi: 10.1016/S2589-7500(23)00136-X https://doi.org/10.1016/S2589-7500(23)00136-X PMid:37770335 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER