Effects of phototherapy on blood pressure and hemodynamic status in women with rheumatoid arthritis
ORIGINAL RESEARCH ARTICLE
Effects of phototherapy on blood pressure and hemodynamic status in women with rheumatoid arthritis
Article Summary
- DOI: 10.24969/hvt.2025.559
- CARDIOVASCULAR DISEASES
- Published: 06/04/2025
- Received: 26/01/2025
- Revised: 18/03/2025
- Accepted: 18/03/2025
- Views: 4042
- Downloads: 2312
- Keywords: Cardiac output, heart rate, hemodynamic status, phototherapy, rheumatoid arthritis, retrospective study, cohort study
Address for Correspondence: Hugo Mendieta Zeron. Faculty of Medicine, Autonomous University of the State of Mexico, Paseo Tollocan, s/n, Moderna de la Cruz, 50180, Toluca, Mexico
E-mail: drmendietaz@yahoo.com Phone: +52- 722 217 4831
ORCID: Angeles Leyda Aviles García - 0000-0003-4167-686X; Mariana Guadalupe Pineda Gonzalez- 0000-0003-1013-9500; María Jose Vargas Contreras - 0000-0001-5390-5362; Irma Socorro Gonzalez Sanchez - 0009-0003-1659-4042;
Araceli Consuelo Hinojosa Juarez - 0000-0002-9100-9259; Joel Alberto Vargas Hernandez -0000-0001-7404-3132;
Hugo Mendieta Zeron - 0000-0003-3492-8950
Angeles Leyda Aviles García1, Mariana Guadalupe Pineda Gonzalez1, María Jose Vargas Contreras1, Irma Socorro Gonzalez Sanchez2, Araceli Consuelo Hinojosa Juarez1 , Joel Alberto Vargas Hernandez1, Hugo Mendieta Zeron1,2
1Faculty of Medicine, Autonomous University of the State of Mexico, Toluca, 50180, Mexico
2Maternal Perinatal Hospital “Monica Pretelini Saenz", Paseo Tollocan Pte. 201, Universidad, 50010, Toluca, Mexico,
Abstract
Objective: To evaluate the chronic effect of phototherapy on blood pressure and hemodynamic status in women with rheumatoid arthritis.
Methods: In this descriptive and retrospective study, hemodynamic variables of rheumatoid arthritis patients treated with phototherapy within a range between 425 and 650 nm, 11.33 J/cm2, 30 cm above the chest were registered for six months.
Results: Thirty-on patients (mean age 45.4 (16.2) years) were included. There was a significant reduction in heart rate (p=0.02912), cardiac output (p=0.0384), thoracic fluid content (p =0.00165) and thoracic fluid content index (p=0 .00049) from the basal point to the six-month period of follow-up.
Conclusions: Phototherapy could potentially affect the hemodynamic variables in a positive and beneficial effect in patients with rheumatoid arthritis.
Key words: Cardiac output, heart rate, hemodynamic status, phototherapy, rheumatoid arthritis, retrospective study, cohort study
Introduction
Under physiological conditions, endothelial cells are the main regulators of arterial tone homeostasis and vascular growth, sensing and transducing signals between tissue and blood. Disease risk factors can lead to an imbalance in their homeostasis, known as endothelial dysfunction (1). Following this line of knowledge, red and near-infrared light can interact with the cells and modulate their metabolism by interacting with cytochromes in mitochondria, leading to increased oxygen consumption, adenosine tri-phosphate (ATP) and reactive oxygen species (ROS) production, as well as regulating nitric oxide (NO) release and intracellular Ca2+ concentration. This phenomenon is known as photobiomodulation (2).
Photobiomodulation can modulate endothelial dysfunction, improving inflammation, angiogenesis, and vasodilation. According to some studies, intracoronary irradiation of 808 nm and 18 J (0.2 W, 2.05 cm2) during percutaneous coronary interventions can prevent restenosis, as well as 645 nm and 20 J (0.25 W, 2 cm2) can stimulate angiogenesis (1). However, larger randomized controlled trials are needed.
Some studies have shown that low-level laser therapy is able to induce a photobiological response within cells that modifies the micro- and macrovascular response; this accompanies the evidence showing the systemic effects of intravascular laser blood irradiation (3). There has been satisfactory experience with the use of phototherapy in various autoimmune diseases (4, 5).
Graphical abstract
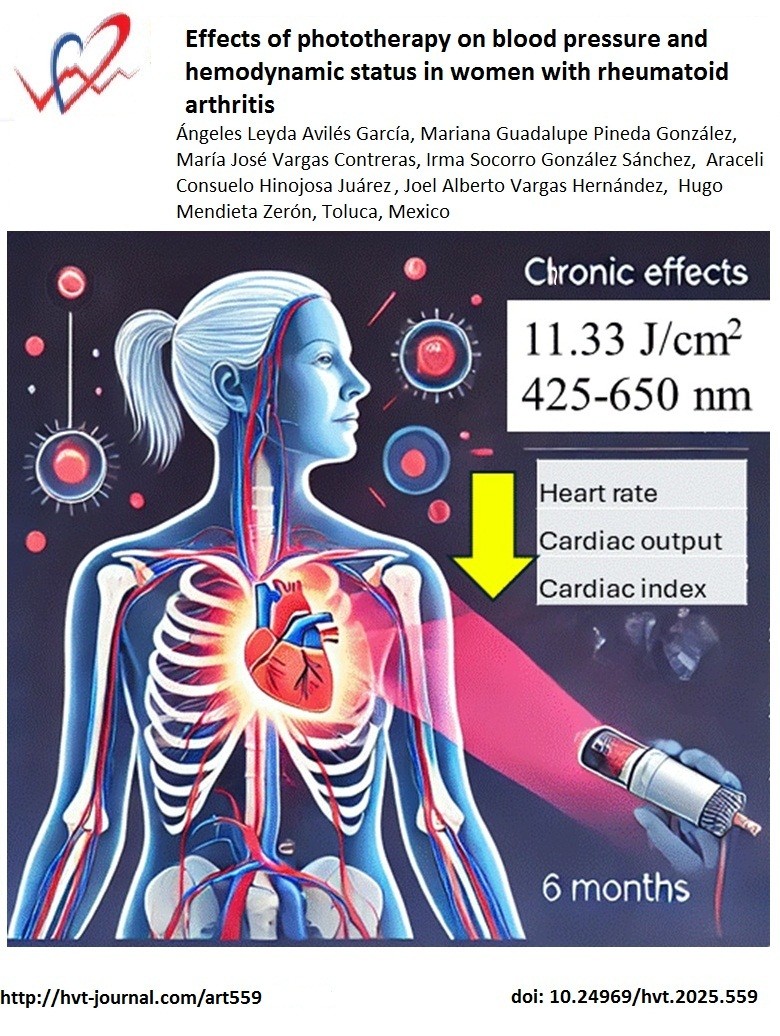
Rheumatoid arthritis is an autoimmune inflammatory disease with complex pathogenesis and with a variable response to different pharmacological management regimens. Due to this and drug cost aspects, there is increasing discussion about phototherapy as a sole or complementary alternative to treat this disease (6). As a matter of fact, phototherapy has been used throughout the history of medicine with various spectrums of ultraviolet (UV) light, laser photodynamic therapy, light-emitting diodes, etc. (7). By contrast, in healthy individuals, a single irradiation with 20 J/cm2 of UVA mobilizes NO from skin stores into the circulation, causes arterial vasodilation, and results in a transient fall in blood pressure (8).
On the other hand, impedance is defined as the resistance to a flow of alternating electric current through a segment. Bioimpedance is dependent on a conductive tissue, which is formed by the intravascular volume composed of the fluids and electrolytes contained in the blood. The impedance in the chest is inversely proportional to the fluid content in the chest (9).
Previous studies with bioimpedance have shown that, in cases of preeclampsia, the following variables are elevated: systolic blood pressure (BP), diastolic BP, mean BP, vascular resistance index, and thoracic flow content (10).
The purpose of this study was to evaluate the chronic effect of phototherapy on hemodynamic status in women with rheumatoid arthritis.
Methods
Study design and population
This was a retrospective cohort study. The medical files of female patients diagnosed with rheumatoid arthritis, treated in the Research Service of the Maternal-Perinatal Hospital “Monica Pretelini Saenz” (HMPMPS), Health Institute of the State of Mexico, in Toluca, Mexico, were reviewed.
Ethical approval has been granted by the local IRB. Patients provided consent for phototherapy.Informed consent of patients for study participation has been waived by the IRB due to the retrospective nature of the study.
Clinical variables and vital signs
Vital signs such as BP heart rate, respiratory rate, and temperature, as well as weight and height, bod ymass index (BMI), were recorded for all patients at the beginning of the study, at three months, and at six months.
We also collected information on medical therapy and comorbidities as diabetes and hypertension.
Phototherapy
The phototherapy lamp (registration number: 1694E95) was placed within a range between 425 and 650 nm, 11.33 J/cm2, 30 cm above the chest. The phototherapy schedule was as follows: a) daily 45-min sessions from Monday to Friday for 2 months; b) three 45-min sessions per week for 2 months; c) 45-min sessions twice a week for 1 month, and d) one session per week for 1 month.
With the patient in the supine position, after recording vital signs (BP, heart rate, respiratory rate, and temperature, as well as weight and height), the phototherapy lamp was placed 30 cm above the chest. The Rheumatoid Arthritis Quality of Life (RAQoL) and the Quality of Life-Rheumatoid Arthritis Scale (QOL-RA) questionnaires were applied to monitor the patients’ clinical evolution.
Thoracic bioimpedance monitoring
It was measured using the Non Invasive Continuous Cardiac Output Monitor (Niccomo) (Medis, Germany), through four bimodal devices, two located on each side of the neck and two on the lateral and inferior side of the thorax. The transmitter emits a high-frequency (60 kHz) and low-amplitude (4 mA) alternating electric current. Blood has the highest electrical conductivity, so the electric current passes primarily through the aorta and then returns to the receiver.
The Niccomo equipment classifies the next parameters: Flow (heart rate, cardiac output, cardiac index, stroke volume, stroke index, systolic BP, diastolic BP, mean arterial pressure), Contractility (velocity index, acceleration index, heather index, pre-ejection period, left ventricular ejection time, systolic time ratio), Fluid (thoracic flow content, thoracic fluid content index), Work (left cardiac work, left cardiac work index) and Vascular (systemic vascular resistance, systemic vascular resistance index, total arterial compliance, total arterial compliance index). Oxygen delivery was also registered.
Statistical analysis
Quantitative variables are represented by measures of central tendency. First, the Kolmogorov- Smirnovtest was performed to determine the normality of the variables. Student’s t-tests or the Mann Whitney U tests were used to do multiple comparisons between groups for all the variables. The l p-value was ≤ 0.05 was accepted as significant.
Results
Information of 31 patients (all women) was recorded (mean age 45.4 (16.2) years). The average time of rheumatoid arthritis progression before starting phototherapy was between 1 and 3 years. The changes in body mass index (BMI), QOL-RA scale and RAQoL scale are shown in Table 1.
|
Table 1. Changes in body mass index, and rheumatoid arthritis scales QOL-RA and RAQoL |
||
|
Variables |
Basal |
Final |
|
BMI, kg/m2 |
25.22 (4.5), 19.2-39.9 |
24.74 (4.6) 17.8-38.2 |
|
QOL-RA scale, points |
13.4 (4.7), 8-30 |
72.6 (9.7), 50-80 |
|
RAQoL scale, points |
28.6 (1.7), 25-30 |
10.5 (9.5) 0-27 |
|
Data are presented as mean (SD), (range) BMI- Body Mass Index, QOL-RA quality of life-rheumatoid arthritis scale, RAQoL - rheumatoid arthritis quality of life, SD - standard deviation |
||
In order of frequency, the medications most used by patients were methotrexate (13), diclofenac (12), folic acid (9), prednisone (8), sulfasalazine (8), celecoxib (4), sulindac (3), deflazacort (3), hydroxychloroquine (3), diacerein (2) and al the rest patients with only one case each one: azathioprine, chloroquine, cyclosporine, leflunomide and meloxicam. In three patients, phototherapy was the only treatment. As comorbidities, systemic arterial hypertension was referred by two patients and type 2 diabetes mellitus was confirmed in one case.
Table 2 shows the mean and standard deviation of all the variables included in the follow-up. After performing the Kolmogorov test it was confirmed a normal distribution of the variables, so the t-test for dependent means was used. According to how the Niccomo classifies the variables, there were three of them that showed statistical changes in the parameter of Flow: reduction of heart rate (p = 0.01294 at three months vs six months and p = 0.02912 baseline vs six months) reduction of cardiac output (p = 0.03177 three months vs six months and p = 0.0384 baseline vs six months) and decrease in cardiac index (p = 0.02395 three months vs six months).; two in the parameter of Fluid – increase in thoracic fluid content rate (p=0.0352 baseline vs three months and p=0.00165 baseline vs six
months] and increase in thoracic fluid content index (p=0.04157 baseline vs three months and p=0.00049 baseline vs six months) and two in the parameter of Work – reduction in left cardiac work (p=0.01356 three months vs six months) and left cardiac work index (p=0.01369 three months vs six months) (Table 3).
Discussion
It has already been suggested that phototherapy including photodynamic therapy and photothermal therapy has demonstrated distinctive potential in rheumatoid arthritis treatment. The suggested mechanism is that under light irradiation, phototherapy can convert light into heat or generate ROS to promote necrosis or apoptosis of rheumatoid arthritis inflammatory cells, thus reducing the concentration of related inflammatory factors and relieving the symptoms of rheumatoid arthritis (11). Besides, there is a clear efficacy of phototherapy in patients who remained non-responsive to previous immunosuppressive therapies (12).
|
Table 2. Mean, standard deviation and range of the included variables |
||||
|
Parameter |
Variables |
Baseline |
Three months |
Six months |
|
Flow |
Heart rate, beats/min |
70.8 (12.0) (51 - 100) |
69.3 (7.7) (53 - 83) |
65.2 (8.8) (47 - 83) |
|
Cardiac output, L/min |
5.3 (1.1) (2.3 – 7.1) |
5.3 (1.2) (3 - 8.1) |
5.0 (1.1) (2.7 - 6.7)) |
|
|
Cardiac index, L/min/m2 |
3.4 (5.9) (1.8 – 4.3) |
3.4 (0.6) (1.9 - 4.3) |
3.2 (0.6 (1.7 - 4.3) |
|
|
SV, mL |
76.9 (19.4) (27 – 107) |
77.3 (18.1 (45 – 119) |
77.4 (19.7) (37 – 112) |
|
|
SI, mL/m2 |
48.6 (10.5) (21 – 64) |
49.2 (8.6 (29 – 67) |
49.7 (10.2) (24 – 62) |
|
|
SBP, mmHg |
109.4 (20.6) (68 – 168) |
109.4 (18.3) (74 – 150) |
110.3 (21.3) (73 – 172) |
|
|
DBP, mmHg |
68.3 (11.5) (44 – 89) |
68.6 (11.7) (43 - 91) |
66.1 (9.8) (46 – 86) |
|
|
MBP, mmHg |
78.6 (13.1) (50 – 105) |
79.3 (12.3) (52 – 103) |
77.5 (10.9) (60 – 110) |
|
|
Contractility |
VI, 1/100 s |
67.2 (22.6) (28 – 116) |
68.3 (19.1) (24 - 104) |
68.9 (21.4) (24 – 109) |
|
AI, 1/100 s2 |
110.7 (43.0) (40 – 223) |
115.4 (36.0) (43 - 189) |
116.1 (40.4) (42 – 200) |
|
|
Heather index, ohm/s2 |
19.2 (6.3) (6.5 - 34.7) |
17.8 (6.6) (1.6 - 31.9) |
17.9 (5.7) (5.4 - 29.3) |
|
|
PEP, ms |
98.5 (26.3) (53 – 215) |
97.9 (26.6) (37 - 199) |
97.5 (18.0) (55 - 126) |
|
|
LVET, ms |
325.6 (41.8) (207 – 396) |
327.2 (36.9) (264 – 404) |
331.6 (40.4) (251 – 402) |
|
|
STR |
0.4 (0.3) (0.1 - 1.3) |
0.3 (0.1) (0.1 - 0.5) |
0.3 (0.1) (0.2 - 0.5) |
|
|
FTc, ms |
4.8 (1.2 ) (2.4 – 7.6) |
4.8 (0.9 ) (3.4 – 7.0) |
5.2 (1.2) (3.5 – 8.3) |
|
|
Fluid |
TFC, kOhm |
30.0 (5.2) (23.5 - 49.1) |
34.3 (11.6) (26.1-87.1) |
32.1 (6.2 (21.7 - 52.4) |
|
TFCI, 1/kΩ*m2 |
19.2 (4.0) (12.1 - 30.6) |
21.9 (7.0) (13.8 - 52.5) |
20.9 (4.6 (13.3 - 31.4) |
|
|
Work |
LCW, kg* m2
|
5.2 (1.3) (3.1 - 8.1) |
5.3 (1.6) (2.3 - 9.4) |
4.8 (1.2) (2.6 - 6.9) |
|
LCWI, kg*m/m2 |
3.3 (0.8) (2.2 - 4.8) |
3.4 (0.9) (2.0 - 5.8) |
3.1 (0.8) (1.7 - 4.4) |
|
|
Vascular |
SVR (dyn • s • cm-5) |
1177.3 (505.4) (508 - 3317) |
1166.3 (371.0) (681 – 2352) |
1220.7 (374.2) (754 – 2134) |
|
SVRI, dyn*s/cm5*m2 |
2014.4 (1170.1) (874 – 7332) |
1792.5 (543.9) (1116 – 3671) |
1860.9 (588) (1051 – 3331) |
|
|
TAC, mL/mm Hg |
2.1 (0.8) (0.4 - 4.1) |
2.1 (0.9) (0.7 - 4.7) |
2.0 (0.9) (0.6 - 4.8) |
|
|
Table 2. Mean, standard deviation and range of the included variables Continued from page…. |
||||
|
|
TACI, mL m2/mm Hg |
1.3 (0.5) (0.3 - 2.4) |
1.3 (0.5) (0.5 - 2.8) |
1.3 (0.6) (0.4 - 2.9) |
|
Others |
DO2, ml O2/min/m2 |
533.7 (108.7) (213 - 703) |
551.8 (95.0) (311 – 701) |
518.7 (98.7) (281 - 690) |
|
Data are presented as mean (SD), (range) AI- acceleration index, DBP- Diastolic blood pressure, DO2- oxygen delivery, FTc- corrected flow time, LCW- left cardiac work, LCWI- left cardiac work index, LVET- left ventricular ejection time, MBP- mean blood pressure, PEP- pre-ejection period, SBP- systolic blood pressure, SI- stroke index, STR- systolic time ratio, SV- stroke volume, SVR- systemic vascular resistance, SVRI- systemic vascular resistance index, TAC- total arterial compliance, TACI- total arterial compliance index, TFC- Thoracic Fluid Content, TFCI- Thoracic Fluid Content Index, VI- Velocity Index |
||||
|
Table 3. Statistical analysis in six months of follow up |
||||
|
Parameter |
Variable |
Baseline vs three months |
Baseline vs six months |
Three months vs six months |
|
Flow |
Heart rate, beats/min |
t = -0.74, p = 0.4655 |
t = -2.29, p = 0.02912 |
t = -2.64, p = 0.01294 |
|
Cardiac output, L/min |
t = -0.17, p =.86561 |
t = 2.14, p = 0.0384 |
t = -2.25, p = 0.03177 |
|
|
Cardiac index, L/min/m2 |
t = -0.94, p =.35539 |
t = -1.14, p =.26199 |
t = -2.38, p =.02395 |
|
|
SV, mL |
t = 0.16, p = 0.87784 |
t = 0.17, p = 0.86734 |
t = 0.17, p = 0.86734 |
|
|
SI, mL/m2 |
t = 0.44, p = 0.66246 |
t = 0.58, p = 0.56303 |
t = 0.34, p = 0.73556 |
|
|
SBP, mmHg |
t = 0, p = 1 |
t = 0.31, p = 0.76028 |
t = 0.31, p = 0.75977 |
|
|
DBP, mmHg |
t = 0.14, p = 0.88716 |
t = -1.32, p = 0.195 |
t = -1.60, p =.12042 |
|
|
MBP, mmHg |
t = 0.33, p = 0.74664 |
t = -0.59, p = 0.56124 |
t = -1.13, p = 0.26679 |
|
|
Contractility |
VI, 1/100 s |
t = 0.45, p = 0.65543 |
t = 0.52, p = 0.60726 |
t = 0.23, p = 0.82065 |
|
AI, 1/100 s2 |
t = 1.01, p = 0.32215 |
t = 1.00, p = 0.32266 |
t = 0.16, p =.8751 |
|
|
Heather index, ohm/s2 |
t = -1.41, p = 0.16844 |
t = 1.53, p = 0.13555 |
t = 0.15, p = 0.88014 |
|
|
PEP, ms |
t = -0.1, p = 0.92113 |
t = -0.16, p = 0.8763 |
t = -0.07, p = 0.94365 |
|
|
LVET, ms |
t = 0.204507, p = 0.83934 |
t = 0.707668, p = 0.48461 |
t = 0.593902, p = 0.55703 |
|
|
STR |
t = -1.41, p = 0.17023 |
t = -1.25, p = 0.22185 |
t = -0.09, p = 0.93168 |
|
|
FTc, ms |
t = -1.17, p = 0.9158 |
t = -1.62, p = 0.11561 |
t = 1.89, p = 0.06827 |
|
|
Fluid |
TFC, kOhm |
t = 2.20, p = 0.0352 |
t = 3.46, p = 0.00165 |
t = -1.15, p = 0.2606 |
|
TFCI, 1/kΩ*m2 |
t = 2.13, p = 0.04157 |
t = 3.91, p = 0.00049 |
t = -0.83, p = 0.41516 |
|
|
Work |
LCW, kg* m2
|
t = 0.41, p = 0.68676 |
t = -1.65, p = 0.1096 |
t = -2.62, p = 0.01356 |
|
LCWI, kg*m/m2 |
t = 0.75, p = 0.45718 |
t = -1.18, p = 0.2487 |
t = -2.62, p = 0.01369 |
|
|
Vascular |
SVR (dyn • s • cm-5) |
t = -0.16, p = 0.87543 |
t = 0.60, p = 0.55518 |
t = 1.20, p = 0.2396 |
|
SVRI, dyn*s/cm5*m2 |
t = -1.02, p = 0.31418 |
t = -0.69, p = 0.49305 |
t = 0.93, p = 0.36012 |
|
|
TAC, mL/mm Hg |
t = 0.262593, p = 0.79466 |
t = 0.298206, p = 0.7676 |
t = -0.546877, p = 0.58851 |
|
|
TACI, mL m2/mm Hg |
t = 0.492137, p = 0.6262 |
t = 0.043639, p = 0.96548 |
t = -0.49699, p = 0.62282ñ |
|
|
Others |
DO2, ml O2/min/m2 |
t = 1.06, p = 0.29631 |
t = -0.89, p = 0.38213 |
t = -2.34, p =0.02617 |
|
t- t-value of the T-test (a t-value of 0 indicates that the sample results exactly equal the null hypothesis. As the difference between the sample data and the null hypothesis increases, the absolute value of the t-value increases). Abbreviations – see Table 2.
|
||||
The clinical meaning of reduction of hemodynamic parameters for patients with rheumatoid arthritis
There is a plenty of information about hemodynamic changes with phototherapy treatment in neonates, and they show changes mainly in cardiac output (decrease in cardiac systolic volume and blood pressure) (13, 14). Especially in preterm newborns, when they receive phototherapy, the decrease in stroke volume leads to a reduction in the cardiac output. The heart rate variability is slightly reduced (as the sympathetic activity predominates). Systemic blood pressure is decreased and heart rate is elevated in both preterm and term newborns during phototherapy (14).
By contrast, there is so little information about hemodynamic changes in adult patients treated with some variant of phototherapy. For example, a previous publication showed that intravascular laser irradiation of blood therapy applied to the primitive carotid artery induces a reduction in blood pressure and, more notably, heart rate in mastectomized women using the tamoxifen or aromatase inhibitors (15).
It is well known that the impedance in the thorax is inversely proportional to the fluid content in the thorax. For example, with each heartbeat, the heart pumps blood into the aorta, which increases the fluid content in the thorax, producing a dramatic decrease in the impedance to the flow of electric current. The behavior of these two variables followed this trend in our study, since while the thoracic flow content increased at three months, at six months it decreased to approach the baseline level, a situation exactly opposite to the impedance. Moreover, previous studies have shown that an increase in thoracic flow content is associated with diastolic dysfunction in patients with heart failure; the behavior shown by this variable in rheumatoid arthritis is not clear (16).
Considering the number of variables per parameter and the measurements in which there was significance, the Fluid parameter would be the most clearly influenced by phototherapy since out of six measurements, there were statistically significant changes in four. By contrast, the Vascular parameter shows no statistically significant change in any time point of measure. The variables that showed the most consistent changes were heart rate and cardiac output.
A strength of this study is that it shows consistency with reported benefits of phototherapy and that it offers an affordable and accessible alternative for most patients if it could be implemented widely.
Study limitations
The fact that this was a retrospective study is a clear limitation for generalizing the findings. It is also evident that the actions of each antirheumatic drug make it difficult to discern the true effect of phototherapy. Notwithstanding, with the results found (reduction in heart rate, cardiac output, cardiac index, O2 supply, left cardiac work and left cardiac work index), it can be suggested that phototherapy reduces the dynamic state of the patients with rheumatoid arthritis.
Conclusion
The hemodynamic status of the patients with rheumatoid arthritis treated with phototherapy within a range between 425 and 650 nm, 11.33 J/cm2, 30 cm above the chest is improved.
Ethics: Ethical approval has been granted by the local IRB. Patients provided consent for phototherapy.
Informed consent of patients to participate in the study has been waived by the IRB due to the retrospective nature of the study.
Peer-review- External and internal
Conflict of interest- None to declare
Authorship- A.L.A.G., M.G.P.G., M.J.V.C., I.S.G.S., A.C.H.J., J.A.V.H., and H.M.Z. contributed equally to the study and preparation of manuscript.
Acknowledgement: We wish to acknowledge the mentorship of Dr. Jose Meneses Calderon, who started the Research Laboratory where this project was performed.
Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material- Do not apply
References
| 1. Colombo E, Signore A, Aicardi S, Zekiy A, Utyuzh A, Benedicenti S, et al. Experimental and clinical applications of red and near-infrared photobiomodulation on endothelial dysfunction- A review. Biomedicines 2021; 9: 274. https://doi.org/10.3390/biomedicines9030274 PMid:33803396 PMCid:PMC7998572 |
||||
| 2. Lipko NB. Photobiomodulation- evolution and adaptation. Photobiomodulation Photomed Laser Surg 2022; 40: 213-33. https://doi.org/10.1089/photob.2021.0145 PMid:35353639 |
||||
| 3. Isabella APJ, Silva JTC, Da Silva T, Rodrigues MFSD, Horliana ACRT, Motta LJ, et al. Effect of irradiation with intravascular laser on the hemodynamic variables of hypertensive patients- Study protocol for prospective blinded randomized clinical trial. Medicine (Baltimore) 2019; 98: e15111. https://doi.org/10.1097/MD.0000000000015111 PMid:30946378 PMCid:PMC6455989 |
||||
| 4. Meneses Calderon J, Gonzalez Sanchez I, Aburto Huacuz G, Alonso Barreto AS, Colín Ferreyra MC, Mendieta Zeron H. Trends of inflammatory markers and cytokines after one month of phototherapy in patients with rheumatoid arthritis. Acta Medica Acad 2015; 44; 102. https://doi.org/10.5644/ama2006-124.137 PMid:26702905 |
||||
| 5. Meneses Calderon J, Aburto Huacuz G, Gonzalez Sanchez I, Gutierrez Vilchis A, Mendieta Zeron H. Phototherapy induces an improvement in clinical and biochemical scores in rheumatoid arthritis. West Indian Med J 2021; 69: 21-5. | ||||
| 6. Zhang Z, Wang R, Xue H, Knoedler S, Geng Y, Liao Y, et al. Phototherapy techniques for the management of musculoskeletal disorders- strategies and recent advances. Biomater Res 2023; 27: 123. https://doi.org/10.1186/s40824-023-00458-8 PMid:38017585 PMCid:PMC10685661 |
||||
| 7. Neupane J, Ghimire S, Shakya S, Chaudhary L, Shrivastava VP. Effect of light emitting diodes in the photodynamic therapy of rheumatoid arthritis. Photodiagnosis Photodyn Ther 2010; 7: 44-9. https://doi.org/10.1016/j.pdpdt.2009.12.006 PMid:20230993 |
||||
| 8. Weller RB, Macintyre IM, Melville V, Farrugia M, Feelisch M, Webb DJ. The effect of daily UVA phototherapy for 2 weeks on clinic and 24-h blood pressure in individuals with mild hypertension. J Hum Hypertens 2022; 37: 548-53. https://doi.org/10.1038/s41371-022-00729-2 PMid:35931819 PMCid:PMC10328825 |
||||
| 9. Ochoa M J, McEwen O JG, Aristizabal O D. Principios de la evaluacion hemodinamica no invasiva con cardiografía de impedancia. Rev Colomb Cardiol 2009; 16: 91-102. | ||||
| 10. Meneses Calderon J, Moreno Santillan AA, Gonzalez Díaz JI, Díaz de Leon Ponce MA, Rodríguez Roldan M, Castorena de Avila R, et al. Medicion hemodinamica en preeclampsia severa. Rev Asoc Mex Med Crit Ter Int 2008; 22: 10-4. | ||||
| 11. Dong Y, Cao W, Cao J. Treatment of rheumatoid arthritis by phototherapy- advances and perspectives. Nanoscale 2021; 13: 14591-608. https://doi.org/10.1039/D1NR03623H PMid:34473167 |
||||
| 12. Miziołek B, Tworek M, Łapczyńska E, Tekielak A, Kochanowska J, Polak K, et al. Utility of phototherapy in patients with systemic sclerosis. Systematic review. Dermatol Ther 2022; 35: e15478 https://doi.org/10.1111/dth.15478 PMCid:PMC9874778 |
||||
| 13. Javorka K, Nandražiova L, Uhríkova Z, Czippelova B, Maťašova K, Javorka M, et al. Cardiovascular changes during phototherapy in newborns. Physiol Res 2022; 71(Suppl. 2): S179-86. https://doi.org/10.33549/physiolres.935002 PMid:36647906 PMCid:PMC9906667 |
||||
| 14. Javorka K, Maťašova K, Javorka M, Zibolen M. Mechanisms of cardiovascular changes of phototherapy in newborns with hyperbilirubinemia. Physiol Res 2023; 72(Suppl 1): S1-9. https://doi.org/10.33549/physiolres.935018 PMid:37294113 PMCid:PMC10292817 |
||||
| 15. Pacheco JA, Molena KF, Veiga EV. Photobiomodulation for blood pressure and heart rate reduction in mastectomized women on hormone blockers- a randomized controlled trial. Photobiomodulation Photomed Laser Surg 2024; 42: 294-305. https://doi.org/10.1089/photob.2023.0136 PMid:38530295 |
||||
| 16. Malfatto G, Branzi G, Giglio A, Villani A, Facchini C, Ciambellotti F, et al. Transthoracic bioimpedance and brain natriuretic peptide levels accurately indicate additional diastolic dysfunction in patients with chronic advanced systolic heart failure. Eur J Heart Fail 2010; 12: 928-35. https://doi.org/10.1093/eurjhf/hfq089 PMid:20562427 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

