Severity of obstructive sleep apnea and its impact on coronary artery disease outcomes: A cross-sectional study in Egypt
ORIGINAL RESEARCH ARTICLE
Severity of obstructive sleep apnea and its impact on coronary artery disease outcomes: A cross-sectional study in Egypt
Article Summary
- DOI: 10.24969/hvt.2025.564
- CARDIOVASCULAR DISEASES
- Published: 11/05/2025
- Received: 19/03/2025
- Revised: 30/04/2025
- Accepted: 01/05/2025
- Views: 3490
- Downloads: 2026
- Keywords: Obstructive sleep apnea, coronary artery disease, polysomnography, STOP-BANG questionnaire, outcome, predictors, diagnostic accuracy
Address for Correspondence: Ahmed G.Bakry. Cardiology Division, Department of Internal Medicine, Qena Faculty of Medicine, South Valley University, Qena, 83523 Egypt
Email: ahmedgbakry@gmail.com Phone: 0201012895996
ORCID: Ahmed G.Bakry - 0000-0002-4581-4927; Hossam Eldin M. Mahmoud - 0000-0003-4973-7639; Ahmed Hussein - 0000-0002-0205-9984; Esraa A. Taha - 0009-0001-2180-5540; Eptehal M. Dongol - 0000-0003-1643-7437
Facebook: Ahmed G. Bakry - @ahmed.bakry.1806; Hossam Eldin M. Mahmoud - @hosam.ismail.96; Ahmed Hussein - @ahmed.aly.9889; Esraa A. Taha - @esraa.elsaied.9843; Eptehal M. Dongol -@doctorwally
X (twitter): Ahmed G. Bakry - @AhmedGBakry
Hossam Eldin M. Mahmoud1a, Ahmed Hussein2, Esraa A. Taha1b, Eptehal M. Dongol1c, Ahmed G. Bakry 1a*
1aCardiology Division, Department of Internal Medicine, 1b Internal Medicine Department and 1cChest Department, Qena Faculty of Medicine, South Valley University, Qena, Egypt
2Cardiology Department, Faculty of Medicine, Sohag University, Sohag, Egypt
Abstract
Objectives: Coronary artery disease (CAD) and obstructive sleep apnea (OSA) share mechanisms such as intermittent hypoxia, inflammation, and sympathetic activation, worsening cardiac function and CAD severity. This study aimed to assess OSA prevalence in CAD patients and its relationship to CAD severity and cardiac function, while evaluating the diagnostic performance of the STOP-BANG questionnaire.
Methods: A cross-sectional observational study was conducted on one hundred CAD patients confirmed by coronary angiography. OSA was diagnosed using the STOP-BANG questionnaire, Epworth Sleepiness Scale (ESS), and polysomnography (apnea-hypopnea index. AHI). CAD severity was measured by the Gensini score. Cardiac function was evaluated by echocardiography, including left ventricular ejection fraction (LVEF), pulmonary artery systolic pressure (PASP), tricuspid annular plane systolic excursion (TAPSE) and ratio of early mitral inflow velocity to early diastolic mitral annular velocity (E/e’). Statistical analyses included correlations and ROC curve analysis.
Results: OSA was present in 34% of patients, with 24% classified as high-risk (AHI ≥15). STOP-BANG score >3.5 had 84.2% sensitivity and 77.4% specificity (AUC 0.83, p<0.001) in diagnosing OSA. High-risk OSA patients had lower LVEF (51.3 (7.0) vs. 57.5 (8.0)%, p=0.007), reduced TAPSE (18.4 (1.6) vs. 20.1(1.9) mm, p=0.022), and higher PASP (33.4(2.4)vs. 27.9 (7.4) mmHg, p=0.005). Diastolic dysfunction (E/e’=12.4 (3.0) vs. 9.2 (2.1), p<0.001) correlated with OSA severity. Gensini scores were higher in OSA patients (60.2 (22.0) vs. 15.9 (8.4), p<0.001) and positively correlated with oxygen desaturation index (ODI). ODI negatively correlated with LVEF and TAPSE.
Conclusion: OSA is prevalent in CAD patients and correlates with worsened cardiac function and increased CAD severity. STOP-BANG score is a useful screening tool. Early detection and management may improve outcomes.
Key words: Obstructive sleep apnea, coronary artery disease, polysomnography, STOP-BANG questionnaire, outcome, predictors, diagnostic accuracy
Introduction
Coronary artery disease (CAD) is a leading cause of death worldwide, claiming millions of lives each year. It occurs due to the buildup of atherosclerotic plaques in the coronary arteries, leading to conditions such as stable angina, unstable angina, myocardial infarction, and sudden cardiac death. The prevalence of CAD differs across regions and is influenced by factors such as lifestyle, genetics, and environmental conditions (1, 2).
Graphical abstract
The Gensini score is a widely used tool to evaluate the severity of CAD by assessing the extent and location of arterial stenosis during coronary angiography (3).
Obstructive sleep apnea (OSA) is a sleep disease characterized by periodic partial or total obstruction of the upper airway during sleep, resulting in intermittent hypoxia. This hypoxia causes sympathetic activation, oxidative stress, and systemic inflammation, which all lead to cardiovascular (CV) diseases like hypertension, stroke, and CAD. The recurrent cycles of nocturnal hypoxia and reoxygenation can also lead to myocardial ischemia, arrhythmias, and plaque instability. The global prevalence of OSA is estimated to range between 6% and 17% in the general population, with significant overlap among individuals diagnosed with CAD. In Egypt, about 14% of the population is affected with OSA. OSA may involve a lot of CV complications. Intermittent hypoxia in individuals with OSA increases oxidative stress, which has the potential to lead to cell injury and dysfunction of endothelial cells. Additionally, hypoxia-induced sympathetic activation can further lead to high blood pressure and tachycardia, thus enhancing the mechanical load on the CV system. Systemic inflammation of OSA promotes atherosclerosis and contributes to plaque formation and development. All these mechanisms taken together act to enhance the risk of CAD and its complications, such as myocardial infarction and sudden cardiac death. Beyond its contribution to CV disease, OSA can adversely affect cardiac function (4, 5).
The diagnosis of OSA in clinical practice is often based on subjective assessments in combination with objective tests. The Epworth Sleepiness Scale and the STOP-BANG questionnaire provide simple and practical tools for initial screening. Polysomnography, or PSG, is considered the gold standard diagnostic test for OSA, providing comprehensive information about apnea-hypopnea events, oxygen desaturation, and sleep architecture.
These diagnostic approaches allow the identification of OSA and the determination of its severity, which is important for guiding treatment decisions and predicting clinical outcomes (6).
OSA management typically includes modifications in lifestyle, medical therapies, and even surgical interventions on occasion. To date, positive airway pressure therapy including continuous positive airway pressure (CPAP) remains the mainstay of treatments against OSA. CPAP decreases the frequency of events of apnea-hypopnea, attenuates symptoms, and improves oxygenation. Other effective treatment modalities besides CPAP may also be prescribed to certain patients, such as weight reduction, positional therapy, or oral appliances. Treatment of OSA in CAD patients may lead to an improvement in CV outcomes and quality of life. The interaction between OSA and CAD, in particular, is of interest in view of their shared risk factors and interlinked pathophysiological mechanisms. Obesity, smoking, sedentary behavior, and other life habit factors are involved in both diseases. Both genetic predisposition and environmental exposure play a role in the development and progression of OSA and CAD. Shared determinants suggest opportunities for integrated prevention and management strategies (7).
While the association between OSA and CAD has been established, limited data exist regarding the effect of OSA severity on CAD progression and patient outcomes, particularly in Egyptian populations (8).
This study aimed to assess the prevalence of OSA in CAD patients and its relationship to CAD severity and cardiac function, while evaluating the diagnostic performance of the STOP-BANG questionnaire for OSA.
Methods
Study design and population
This study employed a cross-sectional observational design to investigate the relationship between OSA and CAD patients. The study was designed and reported in accordance with the STROBE guidelines to ensure clarity and completeness.
Study setting
The study was carried out in the Cardiology Department at Qena University Hospital, a tertiary care center located in Upper Egypt. The hospital serves a diverse patient population, providing an ideal setting for evaluating CAD and its associated comorbidities.
Study participants
Patients diagnosed with CAD confirmed through coronary angiography were recruited consecutively between January 2023 to August 2024. Exclusion criteria included non-CAD patients, recent revascularization, heart failure (left ventricular ejection fraction, LVEF < 40%), significant valvular disease, significant arrhythmias (atrial fibrillation, frequent ventricular ectopy (>1000/day), significant supraventricular tachycardia), pulmonary diseases (chronic obstructive pulmonary disease, interstitial lung diseases), neurological disorders affecting respiratory control, end-stage renal disease, uncontrolled diabetes (glycosylated hemoglobin HbA1c > 10%), obesity hypoventilation syndrome (body mass index, BMI > 40 kg/m² with hypercapnia), current CPAP/bidirectional positive airway pressure use, chronic inflammatory diseases, active malignancy, alcohol or drug abuse, and inability to complete study procedures. These criteria ensure a homogenous sample by excluding conditions that independently affect cardiac function or sleep apnea severity.
Ethical considerations
The study was approved by South Valley University’s Ethics Committee on Research on Humans. Written informed consent was obtained from all participants (Ethical approval code: SVU/MED/MED018/1/21/3/144). The study followed the ethical guidelines of the Declaration of Helsinki.
Baseline Variables
We assessed demographic (age and sex), anthropometric (body mass index, BMI and neck circumference), risk factors of CAD as smoking, diabetes, hypertension and dyslipidemia.
OSA diagnosis
OSA diagnosis was established using:
• STOP-BANG Questionnaire: An eight-item screening tool for OSA, assessing snoring, tiredness, observed apnea, hypertension, BMI >35 kg/m², age >50 years, neck circumference >40 cm, and male gender. Each positive answer scores one point. A score >4 indicates high OSA risk, with a sensitivity of 88% for moderate-to-severe and 93% for severe OSA (6).
• Epworth Sleepiness Scale (ESS): measures the subject’s general level of daytime sleepiness. It asks participants to rate their likelihood of dozing off in eight common situations such as sitting and reading, watching television, or being a passenger in a car. Each situation is scored from 0 (no chance of dozing) to 3 (high chance of dozing), producing a total score ranging from 0 to 24. A score higher than 10 indicates excessive daytime sleepiness, suggesting the need for further evaluation for sleep disorders like OSA. The ESS is simple to administer, easy to interpret, and has been widely validated (6).
• Polysomnography (PSG): It is the gold standard for OSA diagnosis. It was conducted using SomnoScreen plus RC Compi Portable Polysomnography (Somnomedics, Germany) equipment. PSG monitors brain activity (electroencephalography), eye movements (electrooculography), muscle tone (electromyography), respiratory effort, oxygen levels, and heart rate (electrocardiography) using electrodes and sensors. These data enable precise identification of sleep stages, apneas, and abnormal movements, yielding metrics like sleep efficiency and apnea-hypopnea index (AHI). OSA was defined as AHI >5 events/hour and categorized into mild (5-15), moderate (15-30), and severe (>30). High-risk OSA was defined as AHI >15 (6). We assessed oxygen desaturation index (ODI).
Cardiac function assessment
Echocardiography was performed using Philips Affinity 70 (USA) machine to measure LVEF, tricuspid annular plane systolic excursion (TAPSE) and pulmonary artery systolic pressure (PASP). Diastolic function parameters including E/A ratio (early-to-late diastolic mitral inflow velocity, reflecting left ventricular (LV) relaxation) and E/e’ (early mitral inflow velocity to early diastolic mitral annular velocity 0 estimate LV filling pressure) were assessed. All measurements were following the American Society of Echocardiography guidelines (9).
Coronary angiography and Gensini score
Coronary angiography was performed under local anesthesia using fluoroscopic guidance and performed via femoral or radial access. Significant stenosis was defined as >70% luminal narrowing. CAD severity was quantified by Gensini score, incorporating both the degree of stenosis and the anatomical significance of affected segments. Each lesion was assigned a score based on its degree of stenosis: 25% = 1 point, 50% = 2 points, 75% = 4 points, 90% = 8 points, 99% = 16 points, and 100% = 32 points. This score was then adjusted by a weighting factor depending on the lesion’s anatomical location: left main coronary artery = 5, proximal left anterior descending artery (LAD) = 2.5, mid LAD = 1.5, distal LAD = 1, proximal left circumflex artery (LCX) = 2.5, mid LCX = 2, distal LCX = 1, right coronary artery = 1, and major first diagonal, obtuse marginal, and posterior descending artery = 1. The total Gensini score was calculated by summing all the weighted lesion scores, offering a complete evaluation of coronary atherosclerosis (10).
Study Size
The sample size of 100 patients was chosen based on expected prevalence rates and to provide adequate statistical power for detecting significant relationships between OSA and CAD severity. To minimize bias, two independent cardiologists blinded to the study context assessed coronary angiography results. Objective diagnostic tools, including PSG, were utilized to ensure accurate measurements.
Statistical analysis
The data were analyzed with SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to determine statistical normality. Categorical data are shown as frequencies and percentages, while continuous variables are represented as mean (standard deviation, SD). For comparison of categorical variables, we used the Chi-square and Fisher's exact tests. For normally distributed continuous variables, we performed an independent t-test. Mann-Whitney U test is used for nonparametric continuous variables. ANOVA or Kruskal-Wallis were performed compare variables between multiple groups. Pearson's correlation coefficient was applied for correlation analysis of normally distributed continuous variables. We used ROC curve analysis for assessment of STOP-BANG questionnaire`s diagnostic performance. Multivariate logistic regression was used to assess independent predictors of severe OSA. A p-value of <0.05 was judged statistically significant.
Results
Baseline demographic, clinical and sleep study data across groups
A total of 100 patients were enrolled. The mean age was 61.3 (8.5) years, and 62% were male. Diabetes was present in 24%, hypertension in 40%, dyslipidemia in 52%, and 31% were current smokers.
The average BMI was 28.5 (6.3) kg/m². OSA was diagnosed in 34% of patients. Compared to non-OSA, the OSA group had a higher proportion of males (p=0.032), higher BMI (p=0.002), and a higher prevalence of dyslipidemia (p=0.025). The high-risk OSA subgroup (AHI ≥ 15, n = 24) had the highest values for BMI (28.7 (4.8)kg/m²), STOP-BANG score (4.4 (0.8)), AHI (22.5 (6.1)), ODI (16.34 (2.5)), and ESS score (7.75 (0.58)), all with p < 0.001.
Other variables including age, smoking, diabetes, and hypertension showed no significant group differences. These findings are summarized in Table 1.
|
Table 1. Baseline demographic, clinical and sleep study data across groups |
||||||
|
Variables |
Total (n=100) |
Non-OSA (n=66) |
OSA (n=34) |
Low-risk OSA (n=10) |
High-risk OSA (n=24) |
p |
|
Age, years |
61.3 (8.5) |
62.8 (8.9) |
65.1 (9.2) |
64.1 (7.7) |
66.2 (8.9) |
0.224 |
|
Male, % |
62 (62) |
36 (54.5) |
26 (76.5) |
7 (70) |
19 (79.2) |
0.032* |
|
BMI, kg/m² |
28.5 (6.3) |
26.4 (3.2) |
28.6 (4.9) |
26.4 (2.4) |
28.7 (4.8) |
0.002*; <0.001** |
|
Smoking, n(%) |
31 (31) |
21 (31.8) |
10 (29.4) |
2 (20) |
8 (33.3) |
0.805 |
|
Hypertension, n(%) |
40 (40) |
22 (33.3) |
18 (52.9) |
3 (30) |
15 (62.5) |
0.058 |
|
Diabetes Mellitus, n(%) |
24 (24) |
15 (22.7) |
9 (26.5) |
1 (10) |
8 (33.3) |
0.678 |
|
Dyslipidemia, n(%) |
52 (52) |
29 (43.9) |
23 (67.6) |
5 (50) |
18 (75) |
0.025* |
|
AHI, events/hour |
7.4 (8.6) |
3.2 (0.5) |
18.9 (7.8) |
10.1 (2.3) |
22.5 (6.1) |
<0.001** |
|
ODI, events/hour |
9.3 (6.6) |
3.18 (0.47) |
12.26 (1.1) |
8.26 (3.45) |
16.34 (2.5) |
<0.001** |
|
STOP-BANG score |
2.9 (1.2) |
2.5 (1.0) |
3.7 (1.3) |
2.0 (0.0) |
4.4 (0.8) |
<0.001** |
|
ESS score |
7.0 (0.7) |
6.41 (0.53) |
7.2 (0.57) |
6.81 (0.62) |
7.75 (0.58) |
<0.001** |
|
Data are presented as Mean (SD) for continuous variables or as number (n) and percentage (%) for categorical variables P values indicate statistical significance of differences across groups (Non-OSA, Low-Risk OSA, High-Risk OSA), derived from ANOVA or Kruskal-Wallis tests for continuous variables and Chi-square or Fisher’s exact tests for categorical variables: * - comparison non-OSA vs OSA groups, ** - comparison of high-risk OSA with non-OSA and low risk OSA AHI - apnea-hypopnea index, BMI - body mass index, ESS - Epworth Sleepiness Scale, ODI - oxygen desaturation index, OSA – obstructive sleep apnea, STOP-BANG score – Snoring, Tiredness, Observed apnea, Pressure (blood), Body mass index, Age, Neck circumference, Gender score |
||||||
STOP-BANG screening tool diagnostic performance for OSA
The STOP-BANG score showed a high predictive value for OSA, with a cutoff level of > 3.5 yielding: sensitivity of 84.2%, specificity - 77.4%, positive predictive value - 78.8%, negative predictive value - 83.1%, and AUC = 0.83, p < 0.001. High-risk STOP-BANG scores were significantly associated with positive polysomnography results (64% vs. 4% in low-risk scores, p < 0.001). (Table 2 and Fig. 1)
|
Table 2. Diagnostic performance of STOP-BANG score for detecting OSA |
|
|
Variables |
Value |
|
Cut-off value |
> 3.5 |
|
Sensitivity, % |
84.2 |
|
Specificity, % |
77.4 |
|
Positive predictive value, % |
78.8 |
|
Negative predictive value, % |
83.1 |
|
Area under the curve |
0.83 |
|
p |
<0.001 |
|
OSA – obstructive sleep apnea, STOP-BANG score – Snoring, Tiredness, Observed apnea, Pressure (blood), Body mass index, Age, Neck circumference, Gender score |
|
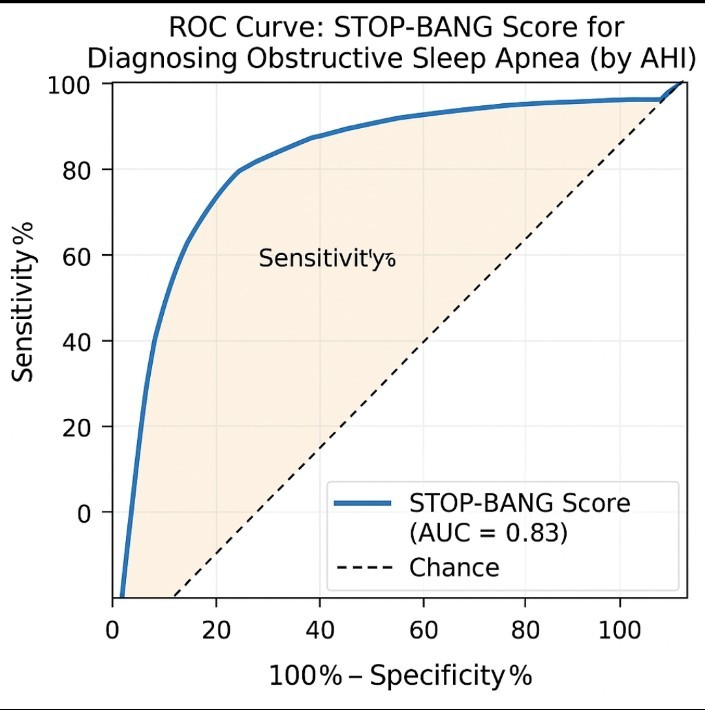
Figure 1. ROC curve showing diagnostic performance of STOP-BANG score in discrimination of patients obstructive sleep apnea as regard AHI
AHI – apnea-hypopnea index, AUC -area under curve
Relationship between OSA and cardiac function
OSA was significantly associated with impaired cardiac function. LVEF was lower in OSA patients compared to non-OSA patients, with the high-risk OSA group exhibiting the lowest LVEF (p = 0.007). TAPSE was significantly lower in the high-risk OSA group compared to non-OSA (p=0.022). PASP was higher in OSA patients than in non-OSA (p = 0.005), with the highest PASP in the high-risk OSA group (33.4 (2.4 mmHg).
E/e’, a marker of diastolic dysfunction, was significantly higher in OSA patients compared to Non-OSA patients (p<0.001), with the high-risk OSA group showing the highest value (12.4 (3.0).
AHI was negatively correlated with LVEF (r= -0.45, p=0.022) and positively correlated with E/e’ (r =0.45, p<0.001). ODI demonstrated a similar pattern, negatively correlating with LVEF (p=0.017) and TAPSE, and positively correlating with PASP (p< 0.001) and E/e’ (r = 0.49, p< 0.001), indicating that more severe OSA correlates with worse cardiac function. These findings are summarized in (Table 3) and illustrated in (Fig. 2 and 3).
|
Table 3. Comparison of CAD severity and cardiac function across OSA groups |
|||||
|
VariableS |
Non-OSA (n=66) |
OSA (n=34) |
High-Risk OSA (n=24) |
Low-Risk OSA (n=10) |
p |
|
Gensini Score |
15.9 (8.4) |
60.2 (22.0) |
58.4 (19.5) |
46.6 (15.2) |
<0.001 |
|
LVEF (%) |
57.5 (8.0) |
52.5 (7.5) |
51.3 (7.0) |
55.1 (7.8) |
0.007 |
|
PASP (mmHg) |
27.9 (7.4) |
32.4 (4.4) |
33.4 (2.4) |
30.1 (6.8) |
0.005 |
|
TAPSE (mm) |
20.1 (1.9) |
18.7 (1.7) |
18.4 (1.6) |
19.5 (1.8) |
0.022 |
|
E/e’ |
9.2 (2.1) |
12.0 (2.9) |
12.4 (3.0) |
11.0 (2.5) |
<0.001 |
|
Data are presented as Mean (SD). The p-values are consistent with a one-way ANOVA or Kruskal-Wallis test comparing the four groups (Non-OSA, OSA, Low-Risk OSA, High-Risk OSA). The choice depends on data normality. CAD –coronary artery disease, E/e’ - ratio of early mitral inflow velocity to early diastolic mitral annular velocity, LVEF - left ventricular ejection fraction, OSA – obstructive sleep apnea, PASP - pulmonary artery systolic pressure, TAPSE - tricuspid annular plane systolic excursion |
|||||
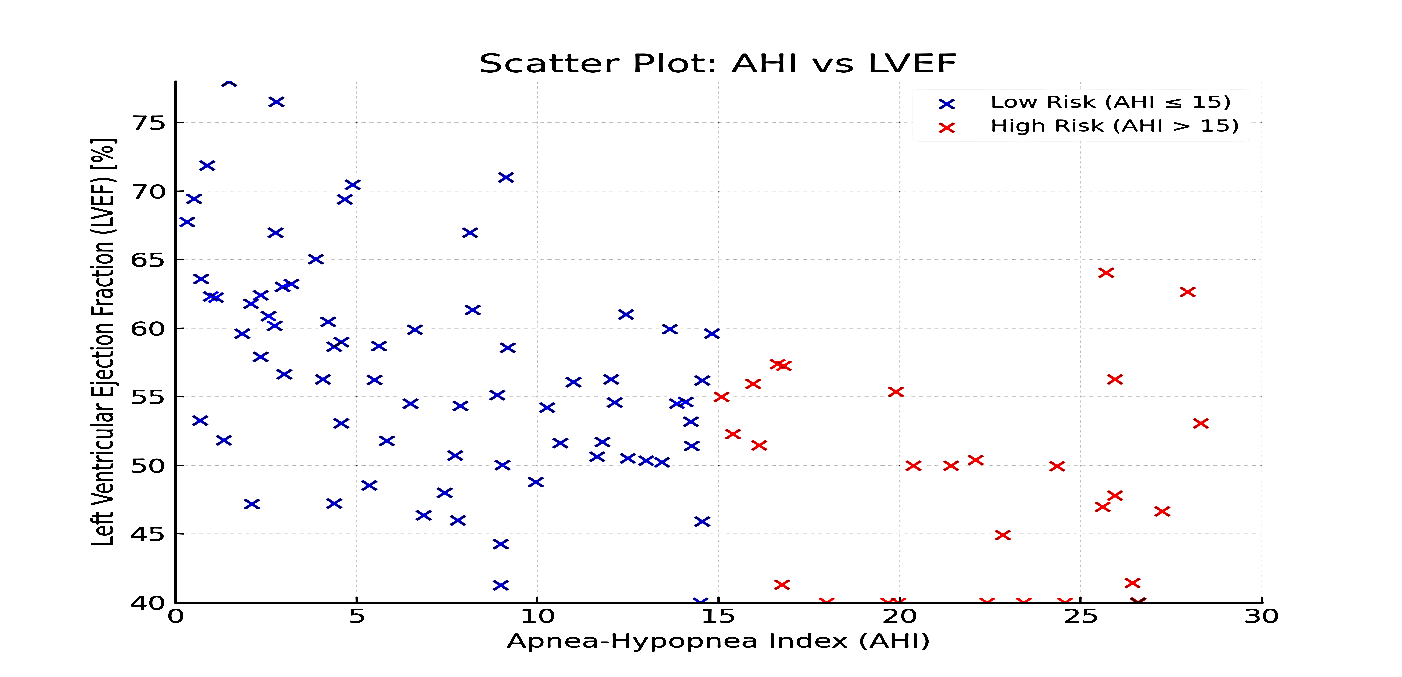
Figure 2. Correlation scatter plots between AHI and LVEF. Scatter plot shows correlation between AHI and LVEF. AHI was negatively correlated with LVEF (r = -0.45, p = 0.022) using Pearson correlation.
AHI – apnea-hypopnea index, LVEF – left ventricular ejection fraction
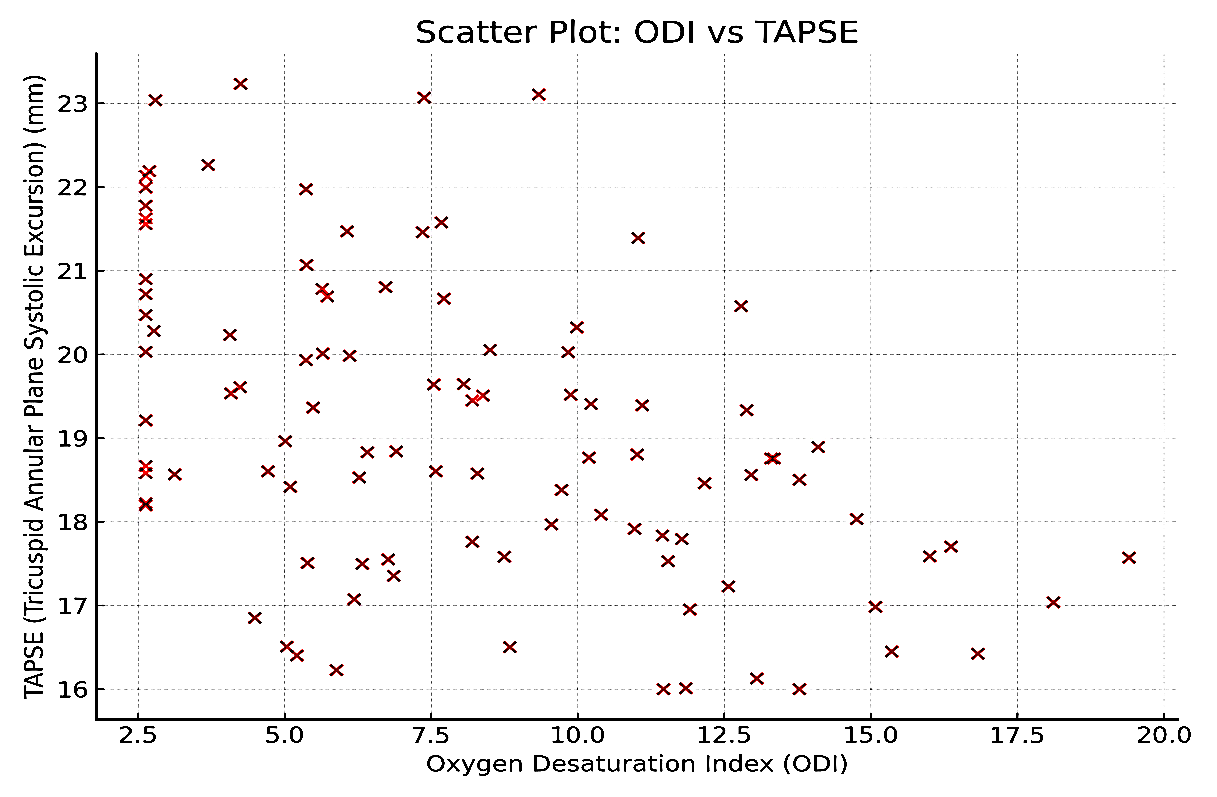
Figure 3. Scatter plot shows correlation between ODI and TAPSE. ODI was negatively correlated with TAPSE (p < 0.001) using Pearson correlation
ODI – oxygen desaturation index, TAPSE - tricuspid annular plane systolic excursion
Relationship between OSA and CAD severity assessed by Gensini score
The mean Gensini score was significantly higher in patients with OSA (60.2 (22.0) compared to those without OSA (15.9 (8.4) (p < 0.001). Within the OSA group, high-risk patients had higher scores (58.4 (19.5) than low-risk patients (46.6 (15.2). The overall group comparison showed a statistically significant difference (p< .001). ODI was positively correlated with Gensini score (p < 0.001), supporting the link between OSA severity and CAD burden. These results are presented in (Table 3).
Multivariable regression analysis
To evaluate independent predictors of high-risk OSA (AHI ≥15) and its association with CAD severity (Gensini Score), we performed multivariate logistic and linear regression analyses. Variables included demographics (age, sex), comorbidities (hypertension, diabetes, dyslipidemia), anthropometrics (BMI, neck circumference), polysomnography parameters (AHI, ODI), and echocardiographic markers (LVEF, TAPSE, E/e') (Tables 4 and 5).
BMI, neck circumference, AHI, dyslipidemia, and diastolic dysfunction (E/e') independently predicted high-risk OSA. AHI, ODI, reduced LVEF, diastolic dysfunction, and smoking were significantly associated with higher Gensini Scores (Table 4). Each 5-unit increase in AHI raised the Gensini Score by 8.21 points, ODI > 20 events was associated with increase in Gensini score by 12.34 points underscoring OSA's role in CAD progression (Table 5).
|
Table 4. Multivariate logistic regression for high-risk OSA |
|||
|
Variables |
Adjusted OR |
95% CI |
p |
|
BMI ≥30 kg/m² |
3.12 |
1.45–6.71 |
0.004 |
|
Neck Circumference >40 cm |
2.87 |
1.32–6.24 |
0.008 |
|
AHI ≥15 events/hour |
4.55 |
2.11–9.83 |
<0.001 |
|
Dyslipidemia |
1.98 |
1.02–3.85 |
0.044 |
|
E/e' >14 |
2.34 |
1.15–4.76 |
0.019 |
|
AHI - apnea-hypopnea index, BMI - body mass index, CI- confidence Interval E/e’ - ratio of early mitral inflow velocity to early diastolic mitral annular velocity, OR -odds ratio, OSA- obstructive sleep apnea |
|||
|
Table 5. Linear regression for CAD Severity (Gensini Score) |
|||
|
Variables |
β coefficient |
Standard error |
p |
|
AHI (per 5-unit increase) |
8.21 |
1.95 |
<0.001 |
|
ODI ≥20 events/hour |
12.34 |
3.12 |
<0.001 |
|
LVEF <50% |
6.78 |
2.45 |
0.007 |
|
E/e' >14 |
9.45 |
2.89 |
0.001 |
|
Current Smoking |
5.12 |
2.11 |
0.016 |
|
AHI - apnea-hypopnea index, ODI - oxygen desaturation index, LVEF - left ventricular ejection fraction, E/e’ - ratio of early mitral inflow velocity to early diastolic mitral annular velocity |
|||
Discussion
OSA was present in 34% of our CAD patients, consistent with reported rates in similar populations. This underscores the importance of early OSA detection, given its role in worsening CAD through ischemia, oxidative stress, and inflammation (4).
Given the high OSA prevalence in CAD patients, routine screening is essential. Tools like the STOP-BANG questionnaire, linked to AHI and worse cardiac outcomes, can identify high-risk patients.
Our study found that higher AHI and ODI correlated with reduced LVEF and TAPSE, indicating impaired ventricular function. OSA's negative impact on cardiac function likely stems from its effects on hemodynamics, ischemia, and arrhythmias.
In concordance with our results, Abd Elghany et al. (11) found a strong association between OSA and cardiac dysfunction in 30 individuals with a mean BMI of 40.1 kg/m² and an average AHI of 22.1 events/hour. Hypertension and diabetes were common in 50% and 30% of patients, respectively. Severe OSA led to deteriorating ventricular function, as evidenced by changes in left ventricular end-diastolic and systolic dimensions, ejection percent, and fractional shortening (p < 0.015). AHI positively correlated with right and left ventricular Tei indices (r = 0.447 and r = 0.391; p < 0.005), highlighting OSA's role in ventricular dysfunction.
In the same line, Oliveira, et al. (12) found that OSA significantly impacts ventricular diastolic function and left atrial (LA) remodeling. Patients with OSA had reduced mitral annular early diastolic velocity, indicating impaired left ventricular diastolic function. The severity of OSA correlated with increased LA volume, reflecting the burden of diastolic dysfunction. The AHI and E/E′ ratio were independent predictors of LA remodelling, linking OSA severity to ventricular dysfunction and associated CV risks (12).
The Gensini score, used to assess CAD severity, correlates with OSA severity in our study. Patients with moderate-to-severe OSA had higher Gensini scores, indicating greater atherosclerotic burden and more severe lesions. This link is driven by OSA-related hypoxia, oxidative stress, and inflammation, which worsen endothelial dysfunction and accelerate CAD progression. OSA severity, measured by AHI, is an independent predictor of higher Gensini scores, highlighting the need to evaluate and manage OSA in CAD patients (13).
In harmony with the current study, Ishiwata, et al. (14) included 98 patients to assess the relationship between OSA severity and CAD, as quantified by the SYNTAX score. Patients with moderate-to-severe OSA (AHI ≥ 15/h) had significantly higher SYNTAX scores compared to those with mild OSA (AHI < 15/h), with a p=0.001. These findings underscore that the severity of OSA is an independent predictor of CAD severity, suggesting that OSA exacerbates the development of atherosclerosis and worsens CAD outcomes.
Also, Mo et al. (15) used polysomnography and coronary computed tomography angiography to study the relationship between OSA and coronary plaque load. Among 119 patients, severe OSA (AHI ≥ 30 events/h) was independently associated with considerable coronary plaque load (p = 0.01). These findings suggest that OSA severity, rather than hypoxemia markers, contributes more to coronary atherosclerosis (15).
Salari et al. (16) conducted a meta-analysis of 24 cohort studies and found that OSA significantly increases the risk of CV diseases, with odds ratios of 1.71 for CV disease, 1.86 for stroke, and 1.77 for mortality. These results indicate that OSA is a notable CV risk factor, associated with higher risks of heart attack, coronary heart disease, and death from CV disease (16).
In agreement with our findings, Strausz, et al. (17) employed a longitudinal study of 36,963 patients and showed that OSA significantly increases the risk of CAD (HR=1.36), type 2 diabetes (HR=1.48), and diabetic kidney disease (HR=1.75). The impact of OSA is notably higher in women, with a hazard ratio of 2.01 for CAD (17).
In agreement with our results, Zhang, et al. (18) investigated the correlation between CAD and OSA in 84 patients, showing that 80% of CAD patients had OSA compared to 61.76% in the control group (18).
Our study shows that OSA is common among CAD patients and is associated with worse cardiac function and more severe coronary lesions. STOP-BANG score proved a reliable screening tool. The results are consistent with prior findings linking OSA severity to CAD severity, diastolic dysfunction, and ventricular remodeling.
OSA’s impact through intermittent hypoxia, oxidative stress, and sympathetic activation explains these observations. Managing OSA could be critical to improving CV outcomes in CAD patients. The strong diagnostic performance of STOP-BANG emphasizes its role as a first-line screening tool in cardiology settings.
Study limitations and future directions
While this study sheds light on the association between OSA and CAD, its cross-sectional design limits the capacity to establish causal relationships. Also, the sample size is relatively small, which may limit the generalizability of the results. Large longitudinal research is needed to better understand the long-term consequences of OSA on CAD development and clinical outcomes. Furthermore, future longitudinal studies should evaluate the effects of OSA treatment and CPAP on CAD progression and cardiac function.
Conclusion
OSA is a significant risk factor for poor outcomes in CAD patients, contributing to worse cardiac function and more severe coronary artery disease. Early screening for OSA, particularly using the STOP-BANG questionnaire and polysomnography, should be integrated into routine CAD management to improve patient care. Further research is needed to establish the benefits of OSA treatment on CAD progression and long-term outcomes.
Ethics: The study was approved by South Valley University’s Ethics Committee on Research on Humans. Written informed consent was obtained from all participants (Ethical approval code: SVU/MED/MED018/1/21/3/144). The study followed the ethical guidelines of the Declaration of Helsinki
Peer-review- External and internal
Conflict of interest- None to declare
Authorship: H.E.M. M., A.H., E.A.T., E.M.D., and A.G.B. equally contributed to the study
and fulfilled all authorship criteria for publication.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Contact authors. Any sharing should be done according to ethics rules with acknowledgement of source or collaboration
References
| 1. Álvarez Álvarez MM, Zanetti D, Carreras Torres R, Moral P, Athanasiadis G. A survey of sub-Saharan gene flow into the Mediterranean at risk loci for coronary artery disease. Eur J Hum Genet 2017; 25: 472-6. https://doi.org/10.1038/ejhg.2016.200 PMid:28098150 PMCid:PMC5386420 |
||||
| 2. Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S. A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 2019; 234: 16812-23. https://doi.org/10.1002/jcp.28350 PMid:30790284 |
||||
| 3. Wang KY, Zheng YY, Wu TT, Ma YT, Xie X. Predictive value of Gensini score in the long-term outcomes of patients with coronary artery disease who underwent PCI. Front Cardiovasc Med 2022; 8: 778615. https://doi.org/10.3389/fcvm.2021.778615 PMid:35141291 PMCid:PMC8818732 |
||||
| 4. Mohamed-Hussein AAR, Wafy S. Prevalence and risk factors of obstructive sleep apnea syndrome in a population of Upper Egypt. Eur Respir J 2010; 36. | ||||
| 5. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev 2017; 34: 70-81. https://doi.org/10.1016/j.smrv.2016.07.002 PMid:27568340 |
||||
| 6. Talatam RP, Suriyan S, Nagesh NJ, Sreekumar S, Sajeer K, Boban M. Comparison of STOP-BANG questionnaire and Epworth sleepiness scale for screening of obstructive sleep apnoea. J Evol Med Dent Sci 2020; 9: 2700-3. https://doi.org/10.14260/jemds/2020/587 |
||||
| 7. Peker Y, Glantz H, Eulenburg C, Wegscheider K, Herlitz J, Thunström E. Positive airway pressure therapy and cardiovascular events in patients with obstructive sleep apnea and coronary artery disease: a systematic review and meta-analysis of randomized trials. Eur Heart J 2023; 44: 987-96. | ||||
| 8. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021; 144: e56-67. https://doi.org/10.1161/CIR.0000000000000988 PMid:34148375 |
||||
| 9. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32: 1-64. https://doi.org/10.1016/j.echo.2018.06.004 PMid:30282592 |
||||
| 10. Yokokawa T, Yoshihisa A, Kiko T, Shimizu T, Misaka T, Yamaki T, et al. Residual Gensini score is associated with long-term cardiac mortality in patients with heart failure after percutaneous coronary intervention. Circ Rep 2020; 2: 89-94. https://doi.org/10.1253/circrep.CR-19-0121 PMid:33693213 PMCid:PMC7929761 |
||||
| 11. Abd Elghany OSAA, Elessawy AF, Elkhashab KA, Elmorsy AS. Correlation between obstructive sleep apnea and ventricular function: a cross-sectional hospital-based study. Acta Cardiol. 2022; 1-8. https://doi.org/10.1080/00015385.2022.2087267 PMid:35695452 |
||||
| 12. Oliveira W, Campos O, Bezerra Lira-Filho E, Cintra FD, Vieira M, Fischer C, et al. Left atrial volume and function in patients with obstructive sleep apnea assessed by real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2008; 21: 1355-61. https://doi.org/10.1016/j.echo.2008.09.007 PMid:18930630 |
||||
| 13. Tan A, Hau W, Ho HH, Ghaem Maralani H, Loo G, Khoo SM, et al. Associations between OSA severity and coronary artery disease were assessed via Gensini scores. J Clin Sleep Med 2020; 16: 755-62. | ||||
| 14. Ishiwata S, Tomita Y, Ishiwata S, Narui K, Daida H, Kasai T. Association between obstructive sleep apnea and SYNTAX score. J Clin Med 2020; 9: 3314. https://doi.org/10.3390/jcm9103314 PMid:33076434 PMCid:PMC7602636 |
||||
| 15. Mo L, Gupta V, Modi R, Munnur K, Cameron JD, Seneviratne S, et al. Severe obstructive sleep apnea is associated with significant coronary artery plaque burden independent of traditional cardiovascular risk factors. Int J Cardiovasc Imaging 2020; 36: 347-55. https://doi.org/10.1007/s10554-019-01710-w PMid:31637622 |
||||
| 16. Salari N, Khazaie H, Abolfathi M, Ghasempour S, Hosseinian-Far A, Mohammadi M, et al. The effect of obstructive sleep apnea on the increased risk of cardiovascular disease: a systematic review and meta-analysis. Neurol Sci 2021. https://doi.org/10.1007/s10072-021-05765-3 PMid:34797460 |
||||
| 17. Strausz S, Havulinna AS, Tuomi T, Bachour A, Groop L, Mäkitie A, et al. Obstructive sleep apnoea and the risk for coronary heart disease and type 2 diabetes: a longitudinal population-based study in Finland. BMJ Open 2018; 8. https://doi.org/10.1136/bmjopen-2018-022752 PMid:30327404 PMCid:PMC6194468 |
||||
| 18. Zhang J, Song Y, Ji Y, Song Y, Liu S, Liu T, et al. Correlation between coronary artery disease and obstructive sleep apnea syndrome and analysis of risk factors. Exp Ther Med 2018; 15: 4771-6. https://doi.org/10.3892/etm.2018.6070 PMid:29805494 PMCid:PMC5958735 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

