Imaging for prosthetic valve endocarditis and cardiac implantable electronic device related infections/endocarditis
EDITORIALS
Imaging for prosthetic valve endocarditis and cardiac implantable electronic device related infections/endocarditis
Article Summary
- DOI: 10.24969/hvt.2025.565
- CARDIOVASCULAR DISEASES
- Published: 16/05/2025
- Received: 11/05/2025
- Accepted: 11/05/2025
- Views: 3759
- Downloads: 2009
- Keywords: Prosthetic valve infective endocarditis, cardiac implantable electronic device-related infective endocarditis, transthoracic echocardiography, transesophageal echocardiography, cardiac computed tomography, FDG -PET)/CT, statement document
Address for Correspondence: Oben Baysan, Guven Hospital Cardiology Clinic, Ankara, Turkey
E-mail: obbaysan@gmail.com
Oben Baysan1, Ilke Zehra Akyildiz2
1Guven Hospital Cardiology Clinic, Ankara, Turkey
2Private cardiology clinic, Izmir, Turkey
Abstract
Prosthetic valve infective endocarditis and cardiac implantable electronic device-related infective endocarditis (CIED IE) are gaining importance due to an increased usage of those medical devices. Recent expert panel statement on this topic shed light on the pros and cons of various imaging modalities used for their diagnosis. We tried to highlight some important points mentioned in that expert panel statement.
Key words: Prosthetic valve infective endocarditis, cardiac implantable electronic device-related infective endocarditis, transthoracic echocardiography, transesophageal echocardiography, cardiac computed tomography, FDG -PET)/CT, statement document
Infective endocarditis (IE) is a serious cardiac condition with a high mortality rate of 20 % in hospital and 25–30 % at six months (1). IE has various clinical presentations, thus leading to diagnostic difficulties. Native valve endocarditis accounts for 80% of cases. Although prosthetic valve endocarditis (PVE) and cardiac implantable electronic device-related infective endocarditis (CIED IE) are less common, they are important subtypes of IE affecting prosthetic cardiac valves, endocardial surfaces, and cardiac devices. The expanding use of cardiac devices such as pacemakers, implantable cardioverter , prosthetic valves, and left ventricular assist devices seems to be the major underlying factor responsible for the increased incidence of CIED IE in recent years encompassing 10% of all IE cases with an incidence rate of 1.0–1.2% per year and mortality rate of 15% (2, 3).
CIED IE is classified as pocket and systemic infections, typically presenting as vegetations within the device lead and/or heart valve (4). Staphylococcus aureus, Coagulase negative staphylococci (CoNS), Streptococci viridans, and Enterococci are the microorganisms most frequently detected in patients with PVE and CIED IE (4) . Gram negative bacteremia is a rare cause of CIED IE, with a few notable exceptions such as Pseudomonas aeruginosa and Serratia marcescens (4). Some microorganisms, especially Staphylococcus aureus, create a biofilm around CIED leads and cause IE even in the absence of visible vegetations (1, 3).
The diagnosis of IE is mainly based on microbiological evidence (isolation of typical microorganisms in blood cultures) and imaging findings in a patient presenting with appropriate clinical symptoms and signs suggestive of IE. The Duke criteria are the most frequently used clinical tool for IE diagnosis first published in 1994 (5); the latest iteration was introduced in 2023 as the Duke–International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis (6). Microbiological evidence, imaging data, and surgical findings constitute the major IE diagnostic criteria. The use of various minor criteria—ranging from predisposing conditions to findings on physical examination and laboratory tests—has also been recommended in that consensus document (6).
Transthoracic and transesophageal echocardiography (TEE), cardiac computed tomography (CT), cardiac CT angiography, and 18 - fluoro-2-deoxyglucose (FDG) positron emission tomography (PET)/CT are the main imaging modalities used in IE diagnosis. In 2022, Dilsizian et al. (7) published an expert panel statement: “Best Practices for Imaging Cardiac Device–Related Infections and Endocarditis,” which contains recommendations for these modalities on patient preparation, image acquisition, processing, interpretation, and standardized reporting.
We aim to highlight some important points on the imaging of CIED IE and PVE in this editorial.
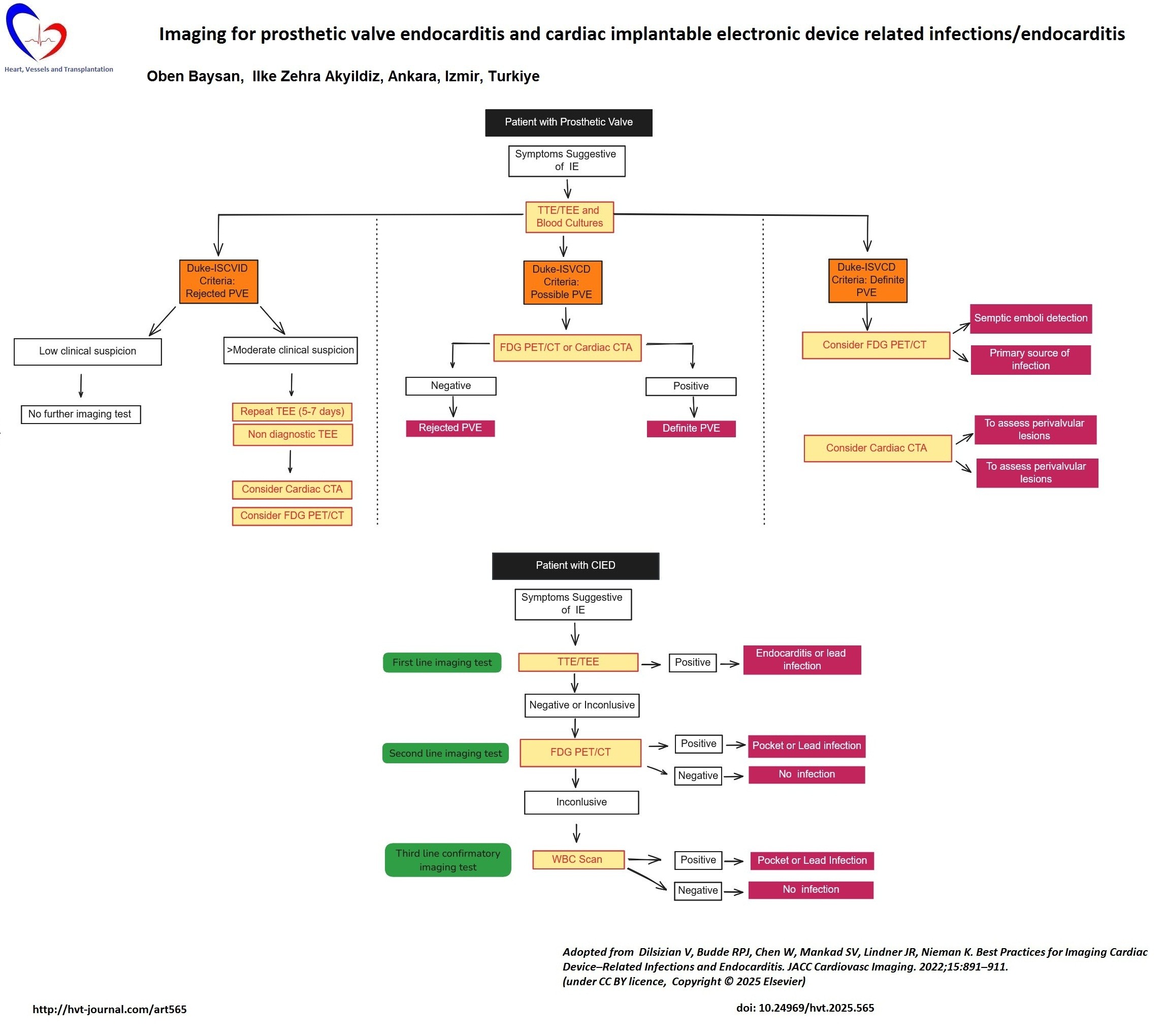
Echocardiography
Transthoracic echocardiography (TTE) should be performed in all patients with suspected cardiac device infection. TTE is a simple and versatile tool for detecting vegetations attached to prosthetic valves or pacemaker leads, prosthetic valve dehiscence, perivalvular abscesses, fistulas, and pseudoaneurysms. It also provides complementary information about prosthetic valve motion, pressure gradients, paravalvular leaks, prosthetic valve regurgitation or stenosis, ventricular function, pericardial effusion, and pulmonary hypertension. Regrettably, TTE has inadequate sensitivity for detecting prosthetic valve vegetations (50%), prosthetic valve abscesses (30–50%), and CIED lead infections (25–40 %) compared with native valve endocarditis(5).
TEE has a much higher sensitivity (> 90 %) than TTE for detecting prosthetic valve vegetations, prosthetic valve abscesses, and lead infections (5). TEE is generally used as a second line test when clinical suspicion of infection is high or the risk of complications is significant even if TTE shows no evidence of endocarditis on prosthetic material or device leads. Alternatively, TEE can also be selected directly as a first line test owing to its higher sensitivity in prosthetic valve or device related endocarditis.
The comprehensive evaluation of any prosthetic valve or device leads by TTE or TEE should be based on a standardized approach (8, 9). In addition to two dimensional and Doppler interrogation; multiplane imaging, X plane imaging, and three dimensional imaging should be used as complementary tools for detecting IE related findings which are vegetations, paravalvular leaks, perivalvular abscesses, pseudoaneurysms, and fistulas.
All echocardiographic findings should be reported in a standardized way. The report should include:
a) Clinical history, indication for imaging, and type of device;
b) Type of echocardiography performed: TTE or TEE;
c) Overall adequacy of the study and specific comment on the adequacy of imaging to assess any prosthetic valves or CIED;
d) Statement of the major findings with respect to abnormal structures or flows;
e) For prosthetic valves, report whether there is evidence of stenosis or regurgitation and whether the latter is transvalvular or paravalvular;
f) Visual comparison with prior studies.
TTE and TEE have limitations arising from false negative and false positive results. A negative echocardiographic study of a prosthetic valve or CIED does not exclude IE. False negative findings usually result from small vegetations, artefacts caused by prosthetic valves that render IE related findings undetectable, difficulty visualizing pacemaker leads, and difficulty differentiating perivalvular or peri-annular abscess from hematoma or annular edema, especially early after surgery.
False positive findings may lead to unnecessary and potentially dangerous treatments which include prolonged antibiotic therapy and device removal. False positive echocardiographic results are usually caused by non-infectious masses resembling vegetations such as thrombi, fibrin strands, and pannus, which are seen in 14% of TEE studies.
Cardiac CT
Cardiac CT is a valuable complementary imaging method because of the limitations of echocardiography described above. Any CT finding suggestive of IE is accepted as an imaging major criterion in the 2023 Duke ISCVID IE criteria (5). CT is more sensitive than TEE for detecting pseudoaneurysms whereas TEE is better for identifying prosthetic valve vegetations.
CT has a limited role in CIED endocarditis. Most prosthetic heart valve types and pacemaker/intracardiac defibrillator leads cause only limited artefacts on CT. In contrast, pacemaker/intracardiac defibrillators generators, shock coils, and Björk-Shiley valves cause extensive artefacts. Moreover, technical issues—such as inhomogeneous contrast enhancement of right heart structures—can prevent satisfactory visualization of pacemaker leads and thus decrease CT sensitivity for detecting CIED endocarditis.
Standard protocols including contrast enhancement and a delayed venous phase acquisition should be used in any prosthetic valve/CIED IE related CT examination. Image acquisition parameters should be optimized. Endocarditis related findings such as vegetations, perivalvular tissue destruction, mycotic aneurysm, abscess formation, and dehiscence on CT should be reported in a standardized way. The report should include:
a) Clinical context and diagnostic question;
b) Procedure: type of acquisition and ECG synchronization; amount of contrast medium and injection protocol; administered pre medication;
c) Comparison: any prior CT for comparison or other studies for correlation;
d) Findings: for valve prostheses, describe vegetations, leaflet or disc motion, and paravalvular dehiscence; for native or bioprosthetic valves, any leaflet thickening. Describe any fistula, abscess, or mycotic aneurysm in terms of size, location, and relationship to vital anatomical structures like coronary arteries. Evaluate the extracardiac anatomy for remote septic emboli, abscesses, pulmonary infections, and other non-infectious findings;
e) Impression: concisely summarize the most important findings and provide clear answers to the clinical questions.
Nuclear imaging
FDG PET/CT
High glucose (FDG) uptake by inflammatory cells can be imaged with PET/CT scanners, which detect early prosthetic valve and CIED infection with high sensitivity. A FDG PET/CT has low sensitivity for native valve endocarditis (31%) (10) and hence negative FDG PET/CT result cannot rule out native valve endocarditis.
TEE or CT based imaging findings in any patient with PVE/CIED do not always provide clear-cut evidence for IE. In such scenarios, FDG PET/CT enables earlier IE diagnosis before morphological changes develop and has a confirmatory role in these cases.
The pooled sensitivity and specificity of FDG PET/CT for PVE and CIED were reported as 73% and 80%, and 87% and 94%, respectively (10). FDG PET/CT is more useful for diagnosing pocket infections than lead infections in CIED: pocket infection sensitivity and specificity are 93% and 98%, whereas lead infection sensitivity is 65% and specificity - 88% (11). In both PVE and CIED lead related endocarditis, adding FDG PET/CT findings to the modified Duke criteria as an additional major criterion increases diagnostic sensitivity from 52–70% to 91–97% without compromising specificity. Whole body FDG PET/CT can also detect remote infectious foci such as spleen. More importantly, FDG PET/CT findings can change clinical management in about 35% of patients.
Inflammation may cause FDG uptake in the postoperative period, but the FDG PET/CT uptake pattern helps differentiate infection from inflammation: uptake is focal at an infection site but homogeneous in an inflammatory area.
A high fat/low carbohydrate diet with overnight fasting should be implemented before FDG PET/CT imaging. As with CT, standardized acquisition protocols should be used. The FDG PET/CT scan should be acquired from the skull vertex to the toes to identify extracardiac infection such as septic embolism. An FDG PET/CT report should include the following points:
a) Clinical history/indication: device type, time of implantation, recent clinical course, laboratory results (WBC count, C-reactive protein), bacterial cultures, antibiotic therapy and duration;
b) Procedure: FDG activity administered (mCi or MBq); injection route (peripheral or central line); uptake time (routine 60 min or delayed); blood glucose level; scan range (whole body); type/volume of iv contrast if a diagnostic CT is acquired;
c) Comparison: any prior PET/CT or other studies—such as TEE or chest CT—for correlation;
d) Findings: presence, location, and distribution of abnormal FDG uptake; other CT findings such as fluid collection, abscess formation, fistulas;
e) Impression: clear diagnosis of infection or non infection.
Radiolabeled white blood cell imaging and gallium 67 citrate
Both radiolabeled white blood cell (WBC) and gallium 67 (67Ga) citrate scanning have lower sensitivity than FDG PET/CT but higher specificity (1). WBC scintigraphy has a role in detecting infection when FDG PET/CT is equivocal and can be used in PVE, CIED or vascular graft infection.
Conclusion
The diagnostic difficulty associated with native valve endocarditis is even more substantial in patients with prosthetic valve endocarditis and cardiac implantable device related device infections. The way to diagnosis starts with patient’s complaints, physical examination findings but they are usually nonspecific and have low sensitivity for the diagnosis. Transthoracic or transesophageal echocardiography has paramount importance for PVE or CIED infection diagnosis. Unfortunately, echocardiography is generally not enough as a sole diagnostic test. Cardiac CT and FDG PET/CT should be used standalone or complementary imaging methods in any patient with PVE and/or CIED infection.
Peer-review- Internal
Conflict of interest- None to declare
Authorship: O.B. and Z.I.A have equally participated in manuscript design and drafting. All authors read and approved the final version, and fulfilled authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Do not apply
References
| 1. Galea N, Bandera F, Lauri C, Autore C, Laghi A, Erba PA. Multimodality imaging in the diagnostic work-up of endocarditis and cardiac implantable electronic device (CIED) infection. J Clin Med 2020; 9: 2237. doi: 10.3390/jcm9072237. https://doi.org/10.3390/jcm9072237 PMid:32674517 PMCid:PMC7408824 |
||||
| 2. Habib G, Lancellotti P, Erba P-A, Sadeghpour A, Meshaal M, Sambola A, et al. The ESC-EORP EURO-ENDO (European Infective Endocarditis) registry. Eur Heart J Qual Care Clin Outcomes 2019; 5: 202-7. doi: 10.1093/ehjqcco/qcz018 https://doi.org/10.1093/ehjqcco/qcz018 PMid:30957862 |
||||
| 3. Bourque JM, Birgersdotter-Green U, Bravo PE, Budde RPJ, Chen W, Chu VH, et al. 18F-FDG PET/CT and radiolabeled leukocyte SPECT/CT imaging for the evaluation of cardiovascular infection in the multimodality context: ASNC Imaging Indications (ASNC I2) Series Expert Consensus Recommendations from ASNC, AATS, ACC, AHA, ASE, EANM, HRS, IDSA, SCCT, SNMMI, and STS. Clin Infect Dis.2024; ciae046. doi: 10.1093/cid/ciae046 https://doi.org/10.1093/cid/ciae046 PMid:38466039 |
||||
| 4. Axell-House DB, Khalil S, Sohail MR. Clinical approach to evaluation of underlying cardiac device infection in patients hospitalized with bacteremia. Methodist DeBakey Cardiovasc J 2023; 19: 48-57. doi: 10.14797/mdcvj.1271 https://doi.org/10.14797/mdcvj.1271 PMid:37547899 PMCid:PMC10402813 |
||||
| 5. Durack DT, Lukes AS, Bright DK, Duke Endocarditis Service. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 1994; 96: 200-9. doi: 10.1016/0002-9343(94)90143-0 https://doi.org/10.1016/0002-9343(94)90143-0 PMid:8154507 |
||||
| 6. Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis 2023; 77: 518-26. doi: 10.1093/cid/ciad271 https://doi.org/10.1093/cid/ciad271 PMid:37138445 PMCid:PMC10681650 |
||||
| 7. Dilsizian V, Budde RPJ, Chen W, Mankad SV, Lindner JR, Nieman K. Best practices for imaging cardiac device-related infections and endocarditis. JACC Cardiovasc Imaging 2022; 15: 891-911. doi: 10.1016/j.jcmg.2021.09.029 https://doi.org/10.1016/j.jcmg.2021.09.029 PMid:34922877 |
||||
| 8. Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr 2013; 26: 921-64. doi: 10.1016/j.echo.2013.07.009 https://doi.org/10.1016/j.echo.2013.07.009 PMid:23998692 |
||||
| 9. Zoghbi WA, Chambers JB, Dumesnil JG, Foster E, Gottdiener JS, Grayburn PA, et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound. J Am Soc Echocardiogr 2009; 22: 975-1014. doi: 10.1016/j.echo.2009.07.013 https://doi.org/10.1016/j.echo.2009.07.013 PMid:19733789 |
||||
| 10. McDonald EG, Aggrey G, Aslan AT, Casias M, Cortes-Penfield N, Dong MQ, et al. Guidelines for diagnosis and management of infective endocarditis in adults: A Guidelines Group Consensus Statement. JAMA Netw Open 2023; 6: e2326366. doi: 10.1001/jamanetworkopen.2023.26366 https://doi.org/10.1001/jamanetworkopen.2023.26366 PMid:37523190 |
||||
| 11.Ten Hove D, Slart RHJA, Sinha B, Glaudemans AWJM, Budde RPJ. 18-FDG PET/CT in infective endocarditis: indications and approaches for standardization. Curr Cardiol Rep 2021; 23: 130. doi: 10.1007/s11886-021-01542-y https://doi.org/10.1007/s11886-021-01542-y PMid:34363148 PMCid:PMC8346431 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

