Problems of early diagnostics of rare demyelinating diseases: A case report
CASE REPORT
Problems of early diagnostics of rare demyelinating diseases: A case report
Article Summary
- DOI: 10.24969/hvt.2025.589
- CARDIOVASCULAR DISEASES
- Published: 02/09/2025
- Received: 12/06/2025
- Revised: 13/08/2025
- Accepted: 13/08/2025
- Views: 1734
- Downloads: 1430
- Keywords: Demilyening diseases, Balo`s concentric sclerosis, neuromyelitis optica spectrum disroders, auquaporin-4 antibodies, multiple sclerosis, pseudotumoral demiyelination, differential diagnosis
Address for Correspondence: Aigerim Sh. Sharabidinova, Department of Neurology and Neurosurgery of the Kyrgyz State Medical Institute of Postgraduate Education and Continuing Education named after S.B. Daniyarov, National Hospital under the Ministry of Health of the Kyrgyz Republic, Bishkek, Kyrgyzstan
E-mail: aigesha.kg@mail.ru
ORCID: Nurzhan T. Dzhaparalieva - 0000-0003-0443-2639; Bolot B.Kulov - 0000-0003-2484-8906; Zhenishbek M.Karimov – XXXX-XXXX-XXXX-XXXX, GJ Osmonova –XXXX-XXXX-XXXX-XXXX, Aigerim Sh. Sharabidinova - XXXX-XXXX-XXXX-XXXX, VM Ysmailova – XXXX-XXXX-XXXX-XXXX
Nurzhan T. Dzhaparalieva1,2, Bolot B.Kulov3, Zhenishbek M. Karimov1,2, G J. Osmonova 4, Aigerim Sh. Sharabidinova1,2, V M. Ysmailova 2
1 Department of Neurology and Neurosurgery of the Kyrgyz State Medical Institute of Postgraduate Education and Continuing Education named after S.B. Daniyarov, Bishkek, Kyrgyzstan
2 National Hospital under the Ministry of Health of the Kyrgyz Republic, Bishkek, Kyrgyzstan
3 Kyrgyz Research Institute of Balneology and Rehabilitation, Tash-Dobo Village, Kyrgyzstan
4 Osh State University, Osh, Kyrgyz Republic
Abstract
Objective: Concentric sclerosis of Balo is a rare demyelinating disease belonging to the group of multiple sclerosis, characterized by a specific "layered" structure of brain damage.
The purpose of the work is to present a case of Balo`s concentric sclerosis and emphasize the importance of timely recognition of rare forms of demyelinating diseases for choosing adequate patient management tactics.
Case presentation: We present a clinical case of a young patient with an acute onset of the disease, occurring with focal neurological symptoms and rapid progression. A clinical and instrumental analysis of a case of Balo's concentric sclerosis in a female patient was performed using a neurological examination, magnetic resonance imaging (MRI) of the brain with contrast, and laboratory studies to exclude other demyelinating and inflammatory diseases
MRI revealed characteristic concentric layered lesions with signs of active inflammation. The clinical picture and dynamics of the disease confirmed the diagnosis of Balo's concentric sclerosis. The patient received immunosuppressive therapy (methylprednisolone) and plasmapheresis, with positive dynamics of neurological symptoms especially restoration of strength in the limbs.
Conclusion: Thus, Balo`s concentric sclerosis presents in female patient with history of viral infection, focal neurological symptoms as hemiparesis and at early stages the imaging may indicate tumor or infection. MRI is informative and shows specific sign for Balo`s concentric sclerosis - layered myelinated and demiyelinated lesions or ``bulls eye`` sign. Immunosuppresive therapy results in improvement of symptoms.
Take-home message: In patients with focal neurological symptoms developed after viral infection and pseudotumor or infection signs on initial imaging studies, repeated MRI imaging helps to establish the diagnosis of Balo`s concentric sclerosis and assist selecting proper therapy.
Key words: Demilyening diseases, Balo`s concentric sclerosis, neuromyelitis optica spectrum disroders, auquaporin-4 antibodies, multiple sclerosis, pseudotumoral demiyelination, differential diagnosis
Introduction
Demyelinating diseases of the central nervous system represent a heterogeneous group of disorders characterized by damage to the myelin sheath of nerve fibers (1). Multiple sclerosis (MS) is the most studied and prevalent form among these disorders. However, there are malignant and atypical variants that occur infrequently, such as neuromyelitis optica spectrum disorders (NMOSD), myelin oligodendrocyte glycoprotein antibody–associated disease (MOGAD), diffuse periaxial encephalitis of Schilder, Baló’s concentric sclerosis (BCS), Marburg’s variant, and pseudotumoral MS (2). These forms are characterized by high variability in clinical course, aggressiveness, distinctive neuroimaging features, and difficulty in differential diagnosis.
Among them, Baló’s concentric sclerosis poses particular clinical and diagnostic challenges.
It is defined by the presence of concentric demyelinating lesions alternating with layers of remyelination, resembling ``tree rings`` or `an onion bulb` on histopathology (1, 3). Since its first description (4), there has been ongoing debate regarding its etiopathogenesis. Various theories have been proposed, including the concentric remyelination hypothesis, the colloid hypothesis, astrocytopathy, and distal oligodendrocytopathy. The discovery that lesions in BCS lack expression of aquaporin-4, one of the astrocytic membrane water channels, provided grounds for suggesting a primary astrocytic role in disease development (6, 7). Another hypothesis proposes that demyelination begins in a central core containing a venule, with demyelinating activity subsequently spreading to the surrounding brain tissue (3).
The true prevalence of BCS remains unknown; it affects men and women equally, most often between the ages of 20 and 50 years. International reports note possible associations with preceding infections and stressful events (2, 6). The literature describes different courses of BCS — asymptomatic, spontaneously regressing, and fulminant. Clinical manifestations vary depending on lesion location, most often including pronounced focal neurological symptoms (paresis, aphasia, cognitive impairment) in combination with generalized brain symptoms (headache, seizures) (5, 8). Most reported cases follow an acute demyelinating syndrome pattern with a monophasic, rapidly progressive course and fatal outcome within weeks or months. However, in recent years, reports have emerged describing longer survival or spontaneous remission (3, 9). Cerebrospinal fluid findings in BCS may include mild lymphocytic pleocytosis with normal protein levels.
The diagnostic challenge lies in the fact that BCS may present as a rapidly progressive brain lesion, often mimicking tumors, neuroinfections, or cerebrovascular accidents (5, 10). Such conditions, particularly in the initial stages, present substantial diagnostic difficulties, requiring a multidisciplinary approach, advanced imaging techniques, and exclusion of multiple other nosologies (7). Despite advances in neuroimaging and immunological diagnostics, rare and atypical demyelinating forms often remain a diagnostic challenge. Early and accurate recognition is critical for appropriate treatment and prevention of irreversible neural damage.
Given the rarity of BCS both in the Kyrgyz Republic and globally, reporting this clinical observation appears justified.
We present a case of Balo`s concentric sclerosis and emphasize the importance of timely recognition of this rare form of demyelinating diseases for choosing adequate patient management tactics.
Case presentation
Patient J., female, 26 years old, was admitted on April 15, 2025, to the Department of Neurology No. 1 of the National Hospital under the Ministry of Health of the Kyrgyz Republic with complaints of weakness in the left extremities, gait disturbance, numbness on the left side of the body, and drooping of the left corner of the mouth.
The disease onset dated to February 25, 2025, when, following an acute respiratory viral infection, the patient experienced numbness of the left thumb, which over the course of a week spread to the index and middle fingers, and subsequently to the left side of the face. On March 12, 2025, brain magnetic resonance imaging (MRI) with contrast enhancement revealed pathological signal-altered lesions in the right frontal and parietal lobes and the left frontal lobe with contrast uptake (neoplasm or neuroinfection could not be excluded). She was evaluated by a neurosurgeon and was prescribed anti-inflammatory and anti-edematous therapy.
Despite treatment, on March 21, 2025, her condition worsened, with numbness in the left arm, weakness in the left extremities, and more pronounced facial asymmetry on the left. Repeated MRI on March 22, 2025, showed lesion enlargement with increased perilesional edema. She received another outpatient treatment course, but numbness persisted, rapidly progressing to the left shoulder, with weakness in the left extremities (more pronounced in the arm) and worsening facial asymmetry.
She was therefore admitted to our hospital for further evaluation:
On physical examination:
No somatic pathology was revealed.
Neurological status examination showed that she was conscious, oriented in time and space, with higher cortical functions intact. Cranial nerves: central-type deficits of cranial nerves VII and XII — smoothing of the left nasolabial fold, drooping of the left corner of the mouth, tongue deviation to the left. Muscle tone normal. Left-sided hemiparesis: muscle strength 3/5 in the arm, 4/5 in the leg. Tendon reflexes brisk on the left, normal on the right. Positive Babinski and Rossolimo signs (left). No meningeal signs. Romberg’s test: mild swaying. Finger-nose test: dysmetria on the left. Sensory: left-sided hemihyperesthesia. Sphincter function preserved. Her EDSS score was 4.0.
Laboratory and instrumental examination data revealed otherwise normal results:
Complete blood count: hemoglobin – 132 g/L, erythrocytes – 4.28 × 10¹²/L, platelets – 264 × 10⁹/L, leukocytes – 7.6 × 10⁹/L, erythrocyte sedimentation rate– 57 mm/h.
Blood biochemistry: alanine aminotransferase – 17.66 U/L, aspartate aminotransferase – 15.30 U/L, creatinine – 77.4 µmol/L, C-reactive protein < 5.0 mg/L, ASO – 600 IU/mL, procalcitonin – 0.11 ng/mL.
Urine analysis: color – pale yellow, clarity – clear, specific gravity – 1.025, protein – negative, leukocytes – 1–2 per high-power field, squamous epithelium – 7–12 per high-power field.
The tests for the viral, infectious and parasitic diseases revealed no significant pathology, except positivity of anti-herpes simplex virus (HSV) Ig G:
Serology: Hepatitis B surface antigen (HBsAg) – negative, hepatitis C virus antigen (anti-HCV) – negative, Wassermann reaction – negative, human immunodeficiency virus (HIV) – negative;
PCR for SARS-CoV-2: negative;
Infectious diseases testing: cytomegalovirus (CMV) immunoglobulin (Ig) M – 0.18 AU/mL, CMV IgG – 250.0 AU/mL, rubella IgG – 8.4 IU/mL, measles IgG – 0.032, measles IgM – negative, tick-borne encephalitis IgM, IgG – negative, Epstein Barr virus (EBV) IgG-Epstein Barr nuclear antigen (EBNA) – 22.0 U/mL, EBV IgM-viral capsid antigen (VCA) – negative, echinococcus IgG – COI = 0.19, toxoplasma IgM – negative, IgG – < 0.2 IU/mL, HSV types 1/2): IgG – positive, IgM – negative;
GeneXpert: Mycobacterium tuberculosis not detected.
Neural antibody panel for infectious, autoimmune, limbic encephalitis and paraneoplastic syndrome were negative:
Neuronal antibody panel: N-methyl- D-aspartate antibodies (NMDA) – < 1:10, anti-contactin-associated-protein-like-2 antibody (CASPR2) – < 1:100, leucine-rich glioma inactivated 1 antibodies (LGI1) – < 1:10, anti-glutamate receptor ½ antiboides (AMPA1/2) – < 1:10, anti-gamma-aminobutyric acid B antibody (GABA-B) – < 1:10.
Tests for connective autoimmune disorder, neuromyelitis optica and antiphospholipid syndrome were negative:
Aquaporin-4 antibodies: < 1:10;
Anticardiolipin antibodies (IgG + A + M): 14.46 U/mL;
Antinuclear antibodies: no fluorescence detected.
The electrocardiogram, chest X-ray and cerebrospinal fluid tests were otherwise normal:
Electrocardiography: sinus rhythm, heart rate – 66 bpm, normal electrical axis;
Chest X-ray: no abnormalities detected;
Cerebrospinal fluid analysis: color – colorless, clarity – clear, cell count – 6 cells/µL, protein – 0.17 g/L, lymphocytes – occasional, glucose – 2.8 mmol/L, chlorides – 106 mmol/L, Pandy and Nonne–Apelt reactions – negative.
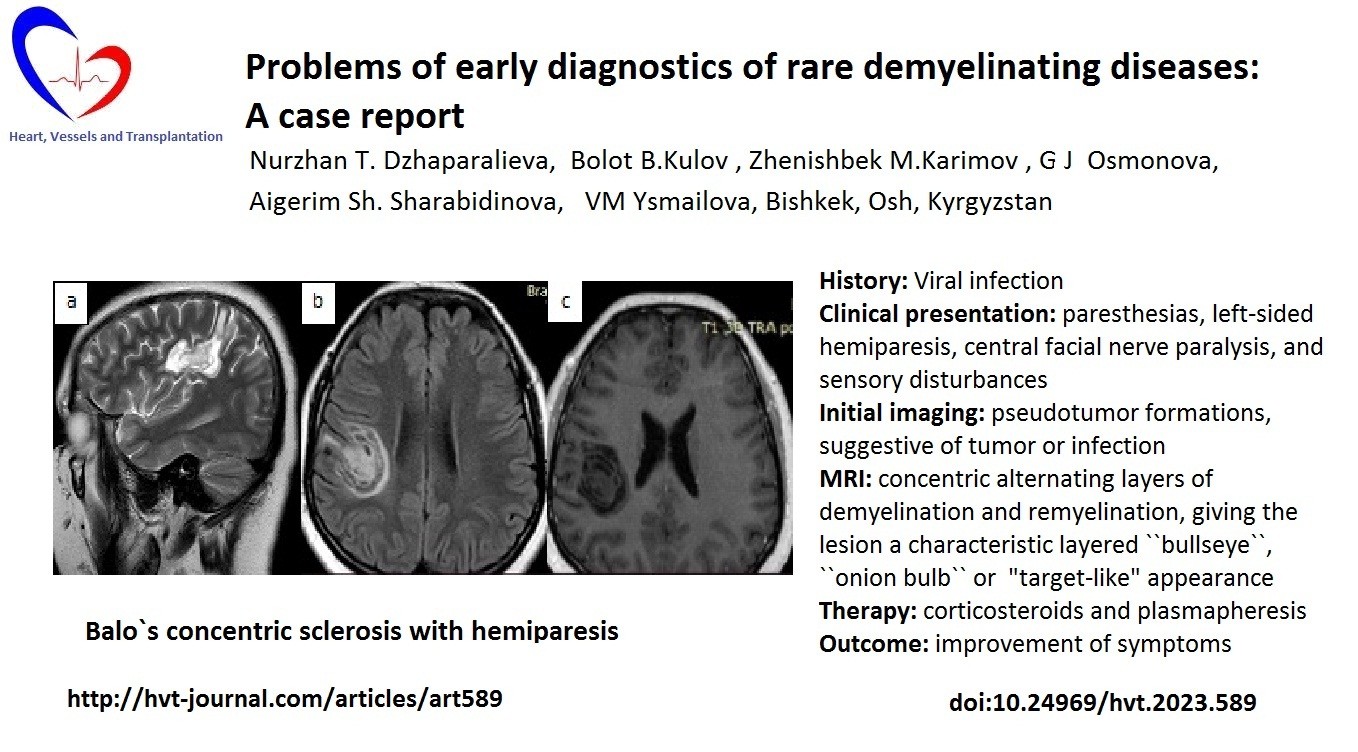
MRI of the brain with contrast from 05/02/2025 showed a concentric demyelinating lesion (in the white matter of the frontal-parietal lobes on the right and the frontal lobe on the left, rounded foci with alternating concentric zones of hyperintense and isointense signal on T2/FLAIR are visualized), with contrast enhancement at the periphery, most likely - concentric Balo sclerosis (Fig. 1, 2).
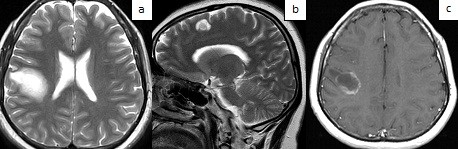
Figure 1. MRI of the brain of patient Zh., 26 years old, with concentric sclerosis of Balo (axial (a, c) and sagittal (b) projections): pathological lesion in the frontal-parietal lobes of the right hemisphere of the brain and parasagittally in the frontal lobe on the left, with ring-shaped enhancement on the post-contrast image
MRI – magnetic resonance imaging
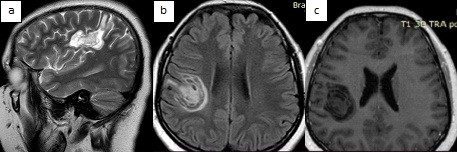
Figure 2. MRI of the brain of patient Zh., 26 years old, with concentric sclerosis of Balo (sagittal (a) and axial (b) projections): in the frontal-parietal lobes of the right hemisphere of the brain and parasagittally in the frontal lobe on the left, foci with characteristic alternating concentric zones of hyperintense and isointense signal
MRI – magnetic resonance imaging
To exclude brain tumor or connective systemic diseases we invited neurosurgeon and rheumatologist for consultation:
• Neurosurgery: no evidence of intracerebral tumor, no indication for surgery;
• Rheumatology: no signs of systemic disease.
Thus, we reached final diagnosis: Baló’s concentric sclerosis with left-sided hemiparesis.
Treatment and outcome: The patient was prescribed high-dose intravenous methylprednisolone (1,000 mg/day for 5 days) followed by oral taper, plus three sessions of plasmapheresis. Clinical improvement was noted — regression of sensory symptoms and increased muscle strength (from 3/5 to 5/5 in the arm, from 4/5 to 4.5/5 in the leg). Long-term immunosuppressive therapy and follow-up are planned.
Discussion
Ballot's concentric sclerosis is a rare variant of demyelinating disease, which can either represent a separate nosological form or be considered within the framework of MS. Since its first description by Joseph Balo in 1928, the disease has remained a subject of scientific debate due to its clinical and pathological uniqueness, as well as diagnostic difficulties, especially at the onset of the disease (1, 4).
The presented case highlights the key problems associated with the detection of BCS in clinical practice and demonstrates the importance of an integrated approach to differential diagnosis. In this patient, the disease began after an acute respiratory viral infection, a trigger often associated with an autoimmune cascade in the pathogenesis of demyelinating disorders. The first symptoms were paresthesias, which over time transformed into pronounced focal neurological symptoms, including left-sided hemiparesis, central facial nerve paralysis, and sensory disturbances. The course of the disease was subacute with a gradual increase in symptoms, which corresponded to acute demyelination syndrome or a tumor process in the brain, but its morphological interpretation was difficult. Early neuroimaging data indicated the presence of space-occupying lesions with contrast accumulation, which was initially interpreted as a possible tumor or infection. This is typical for pseudotumor forms of demyelination, where there are no clear radiological signs that can clearly exclude a neoplasm, especially in the absence of data on previous episodes of demyelination. At this stage, the patient received non-specific anti-inflammatory therapy, without a pronounced clinical response. Only several weeks later, during a repeat MRI assessment, the characteristic feature of BSC was revealed - concentric alternating layers of demyelination and remyelination, giving the lesion a characteristic layered or "target-like" or ``bulls eye`` appearance. This signal pattern on T2/FLAIR with peripheral contrast enhancement is considered pathognomonic for BSC and allowed the correct diagnosis to be formulated. It was dynamic observation, with repeated imaging, that became the decisive diagnostic tool. This emphasizes the importance of repeated high-tech neuroimaging studies in case of diagnostic doubts (6).
Differential diagnostics of BCS requires exclusion of a wide range of pathologies, such as primary and metastatic brain tumors, neuroinfections (viral encephalitis, toxoplasmosis, neurosyphilis), vasculitis, autoimmune encephalitis, ADEM and pseudotumor forms of multiple sclerosis, optic neuromyelitis spectrum disorders and MOGAD-associated diseases (7, 10). In our patient, all the above conditions were consistently excluded based on the results of immunological, serological, microbiological studies, as well as cerebrospinal fluid analysis data, which did not show signs of active inflammation, infection and oncopathology. Negative titers of antibodies to AQP4 and MOG additionally reduced the likelihood of other demyelinating diseases included in the NMOSD and MOGAD spectra.
It is also worth noting that the patient showed a positive response to methylprednisolone pulse therapy and plasmapheresis, which is typical for autoimmune processes and confirms the inflammatory nature of the disease. Improvement in the form of regression of neurological deficit, especially restoration of strength in the limbs, emphasizes the potential reversibility of lesions in the early stages with adequate therapy. From a pathophysiological point of view, there is still no final clarity regarding the exact mechanism of concentric ring formation in KSB. Modern concepts consider a violation of aquaporin-4-dependent astrocyte function, astrocytopathies, as well as metabolic disorders in oligodendrocytes. The absence of aquaporin-4 expression in lesions in BCS makes possible an analogy with neuromyelitis optica spectrum disorders, but in the clinical aspect and immunological profile, these diseases are clearly distinguished (9). It is important to emphasize that the world literature is accumulating data on changes in the stereotypes of the course of BCS: more and more cases are being registered with a relatively benign or monophasic course.
Conclusion: Thus, Balo`s concentric sclerosis may present in female patient with history of viral infection, focal neurological symtpoms as hemiparesis and at early stages the imaging may indicate tumor or infection. MRI is informative and shows specific sign for Balo`s concentric sclerosis - layered myelinated and demiyelinated lesions or ``bullseye`` sign. Immunosuppresive therapy results in improvement of symptoms.
Take-home message: In patients with focal neurological symptoms developed after viral infection and pseudotumor or infection signs on initial imaging studies, repeated MRI imaging helps to establish the diagnosis of Balo`s concentric sclerosis and assist selecting proper therapy.
Ethics: Informed consent was obtained from patient for all procedures
Peer-review: External and internal
Conflict-of-interest: None to declare
Authorship: N.T.Dz., B.B.K., Zh.M.K., G.J. O., A.Sh.Sh,, and V.M.Y. equally contributed to case management, preparation of case report and fulfilled authorship criteria
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declare they did not use A.I. technologies for manuscript preparation
Availability of data and materials: Contact authors. Any share must be in frame of fair use and acknowledgement of source or collaboration
References
| 1.Garshova SV, Ponomarev VV. Concentric sclerosis of Balo: a scientific review and personal observation. Int Neurol J 2017; 5: 23-8. | ||||
| 2.Ripellino P, Khonsari R, Stecco A, Filippi M, Perchinunno M, Cantello R. Clues on Balo's concentric sclerosis evolution from serial analysis of ADC values. Int J Neurosci 2016; 126: 88-95. https://doi.org/10.3109/00207454.2014.989524 PMid:25405537 |
||||
| 3.Lorina LV, Burshinov A O. Concentric sclerosis of Balo: a clinical case of remission. Clinician 2017; 4: 71-5. https://doi.org/10.17650/1818-8338-2016-10-4-71-75 |
||||
| 4 Balo J. Encephalitis periaxialis concentrica. Arch Neur Psych 1928; 19: 242-64. https://doi.org/10.1001/archneurpsyc.1928.02210080044002 |
||||
| 5.Campbell FC, Oti B, Ndafia NM, Hart I, Okwunodulu O, Ndubuisi CA, et al. Balo's concentric sclerosis a rare variant of multiple sclerosis in a Nigerian adult male: A case report. Surg Neurol Int 2022; 13: 486. https://doi.org/10.1016/j.jns.2021.118120 |
||||
| 6.Caracciolo JT, Murtagh RD, Rojiani AM, Murtagh FR. Pathognomonic MR imaging findings in Balo concentric sclerosis. AJNR Am J Neuroradiol 2001; 22: 292-3. | ||||
| 7.Jolliffe EA, Guo Y, Hardy TA, Morris PP, Flanagan EP, Lucchinetti CF, et al.. Clinical and radiologic features, pathology, and treatment of Baló concentric sclerosis. Neurology 2021; 97: e414-22. https://doi.org/10.1212/WNL.0000000000012230 PMid:34011576 PMCid:PMC8362356 |
||||
| 8.Masuda H, Mori M, Katayama K, Kikkawa Y, Kuwabara S. Anti-aquaporin-4 antibody-seronegative NMO spectrum disorder with Balo's concentric lesions. Intern Med 2013; 52: 1517-21. https://doi.org/10.2169/internalmedicine.52.9330 PMid:23812202 |
||||
| 9.Wang C, Zhang KN, Wu XM. Balo's disease showing benign clinical course and co-existence with multiple sclerosis - like lesions in Chinese. J Mult Scler 2008; 14: 418-24. https://doi.org/10.1177/1352458507084036 PMid:18208888 |
||||
| 10.Mihailescu G, Mitrea DA, Vladila AM, Nica SM. Balo's сoncentric sclerosis in a young female patient: case report and review of the literature. J Med Med Sci; 4: 514-9. | ||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

