Staphylococcus aureus infective endocarditis in an adolescent with an initial diagnosis of complicated dengue fever: A case report
CASE REPORT
Staphylococcus aureus infective endocarditis in an adolescent with an initial diagnosis of complicated dengue fever: A case report
Article Summary
- DOI: 10.24969/hvt.2025.590
- CARDIOVASCULAR DISEASES
- Published: 02/09/2025
- Received: 26/06/2025
- Revised: 22/08/2025
- Accepted: 22/08/2025
- Views: 2014
- Downloads: 1408
- Keywords: Embolism, splenic abscess, Staphylococcus aureus, endocarditis, cardiovascular surgery, dengue fever
Address for Correspondence: Nicolas de Albuquerque Pereira Feijoó, Department of Medicine, Universidade do Grande Rio/Afya (UNIGRANRIO/Afya), Barra da Tijuca, Rio de Janeiro 22775-003, Rio de Janeiro, Brazil
Cristiane da Cruz Lamas, Instituto Nacional de Cardiologia, Rio de Janeiro 22240-006, Rio de Janeiro, Brazil; Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, Rio de Janeiro 21040-360, Rio de Janeiro, Brazil
E-mail: nicolasapfeijoo@gmail.com; nicolasfeijoo@unigranrio.com
E-mail: cristianelamas@gmail.com; cristiane.lamas@inc.saude.gov.br
ORCID: Nicolas de Albuquerque Pereira Feijoó – 0000-0002-5065-9933, Thiago Areas Lisboa Netto - 0009-0007-4524-9298, Alina de Souza Santos - 0009-0005-2241-5340, Ellen Fernanda de Neves Braga - 0009-0003-5646-9983, Rafael Quaresma Garrido - 0000-0002-1964-8291, Giovanna lanini Ferraiuoli Barbosa - 0000-0001-9525-7113, Bruno Zappa - 0000-0003-0226-9313, Guilherme Dalcol Torres do Amorim - 0009-0005-5813-0347, Cristiane da Cruz Lamas - 0000-0002-5561-999X
Nicolas de Albuquerque Pereira Feijoó1,2, Thiago Areas Lisboa Netto3, Alina de Souza Santos2, Ellen Fernanda de Neves Braga2, Rafael Quaresma Garrido2,3, Giovanna lanini Ferraiuoli Barbosa2, Bruno Zappa2, Guilherme Dalcol Torres de Amorim2, Cristiane da Cruz Lamas2,3
1Department of Medicine, Universidade do Grande Rio/Afya (UNIGRANRIO/Afya), Barra da Tijuca, Rio de Janeiro 22775-003, Rio de Janeiro, Brazil
2Instituto Nacional de Cardiologia, Rio de Janeiro 22240-006, Rio de Janeiro, Brazil
3Instituto Nacional de Infectologia Evandro Chagas, Fiocruz, Rio de Janeiro 21040-360, Rio de Janeiro, Brazil
Abstract
Objective: To present a case of infective endocarditis (IE) in a 16-year-old male from Rio de Janeiro.
Case presentation: He was initially diagnosed with dengue fever with alarm signs. He developed thrombocytopenia, petechiae and purpura. Staphylococcal bacteremia, meningococcal disease and disseminated gonococcal disease were considered; blood cultures were taken, and empirical vancomycin and ceftriaxone were started. On day 2 of hospitalization, blood cultures were positive for methicillin-sensitive Staphylococcus aureus. Transthoracic echocardiographic revealed mitral valve thickening with the presence of a vegetation (19 x 10 mm). Computed tomography showed splenic abscess. He was transferred to a cardiology referral institute and underwent splenectomy and mitral valve repair with ring insertion. He was discharged on day 54 of hospitalization on cotrimoxazole and rivaroxaban.
Conclusion: This case highlights the importance of differential diagnosis of fever with petechiae and purpura, early initiation of empirical antimicrobials, systematic screening for embolic phenomena in the management of IE and valve repair surgery if feasible.
Keywords: Embolism, splenic abscess, Staphylococcus aureus, endocarditis, cardiovascular surgery, dengue fever
Introduction
Infective endocarditis (IE) is an infrequent disease in the pediatric population. IE by Staphylococcus aureus is a disease with high mortality and high emboligenic potential, whose incidence in the setting of pediatric patients without associated heart disease has been high in retrospective studies (1-5). However, there are few studies on IE in healthy pediatric patients.
We present a case of IE in a young patient with no risk factors for IE, evolving with persistent fever, multiple petechiae and purpura and complicating with embolic phenomena to the spleen, kidneys, central nervous system and lower limbs, who was initially diagnosed with dengue fever.
![]()
Case report
A 16-year-old white, heterosexual male patient student and young apprentice in a supermarket, was admitted to our unit with a history of fever, myalgia, emesis and general malaise for 3 days. He had been previously seen in the Emergency Unit where dengue fever was presumptively diagnosed. He was sent home on analgesia and advised of alarm signs for dengue.
The following day he persisted with fever and developed abdominal pain in the left hypochondrium, and returned to the Emergency unit. Blood count revealed thrombocytopenia (44,000 /mm3). Based on the clinical and laboratory features, he was transferred to a reference unit for infectious diseases.
There was no remarkable personal or family history, his vaccination card was complete for his age, and he denied risky sexual contact. On admission, blood tests were requested and they showed a hemoglobin of 12.1 mg/dl, 5680/mm3 leukocytes (70% neutrophils and 6% rods), a C-reactive protein (CRP) level of 18 mg/dl (reference <0.5 mg/dl) and 66,000/mm3 platelets; rapid tests for dengue NS1 and dengue immunoglobulin (Ig) M and IgG were non-reactive , as were rapid tests for human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV) and syphilis.
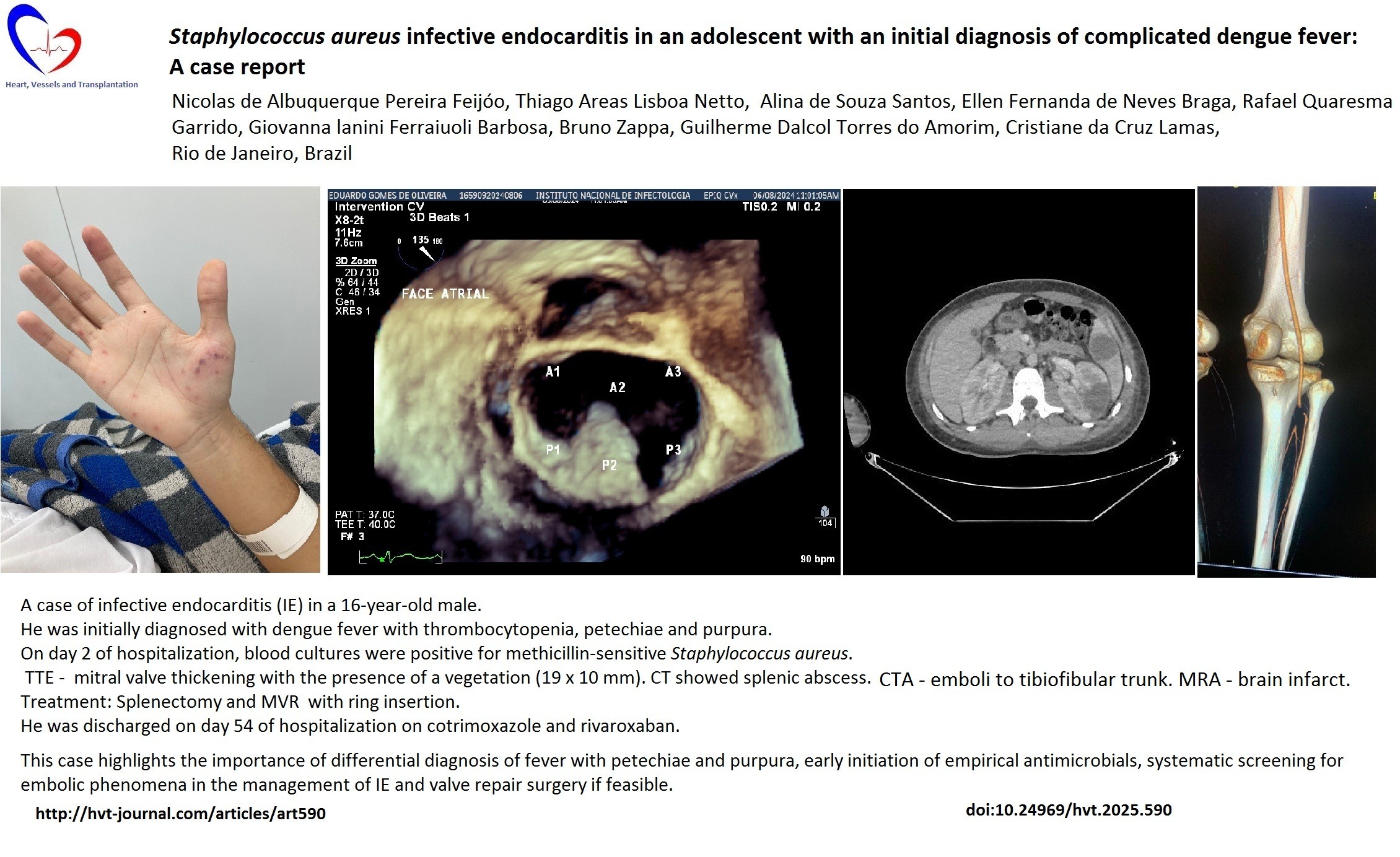
A 3+/6 systolic murmur was identified in the mitral area, he continued to feel poorly, and petechiae and purpura appeared on the right hand on the second day of hospitalization (Fig. 1). Three sets of blood cultures were collected and empirical antimicrobial regimen with vancomycin and ceftriaxone was started. On D3, blood tests showed intense left shift without leukocytosis (4620/mm3 leukocytes with 28% rods) and a CRP of 22 mg/dl.
In less than 24 hours, all blood cultures were positive for Gram-positive cocci later identified in clamps as methicillin-sensitive Staphylococcus aureus, and antibiotic therapy was optimized for oxacillin 2g q4h. Control blood cultures were taken three days after starting the empirical regimen and resulted negative. Computed tomography (CT) of the chest and abdomen without contrast identified pleural effusion at the base of the left hemithorax with adjacent consolidation, hepatomegaly, and splenomegaly associated with extensive hypodense areas, with suspicion of septic embolism to the spleen.
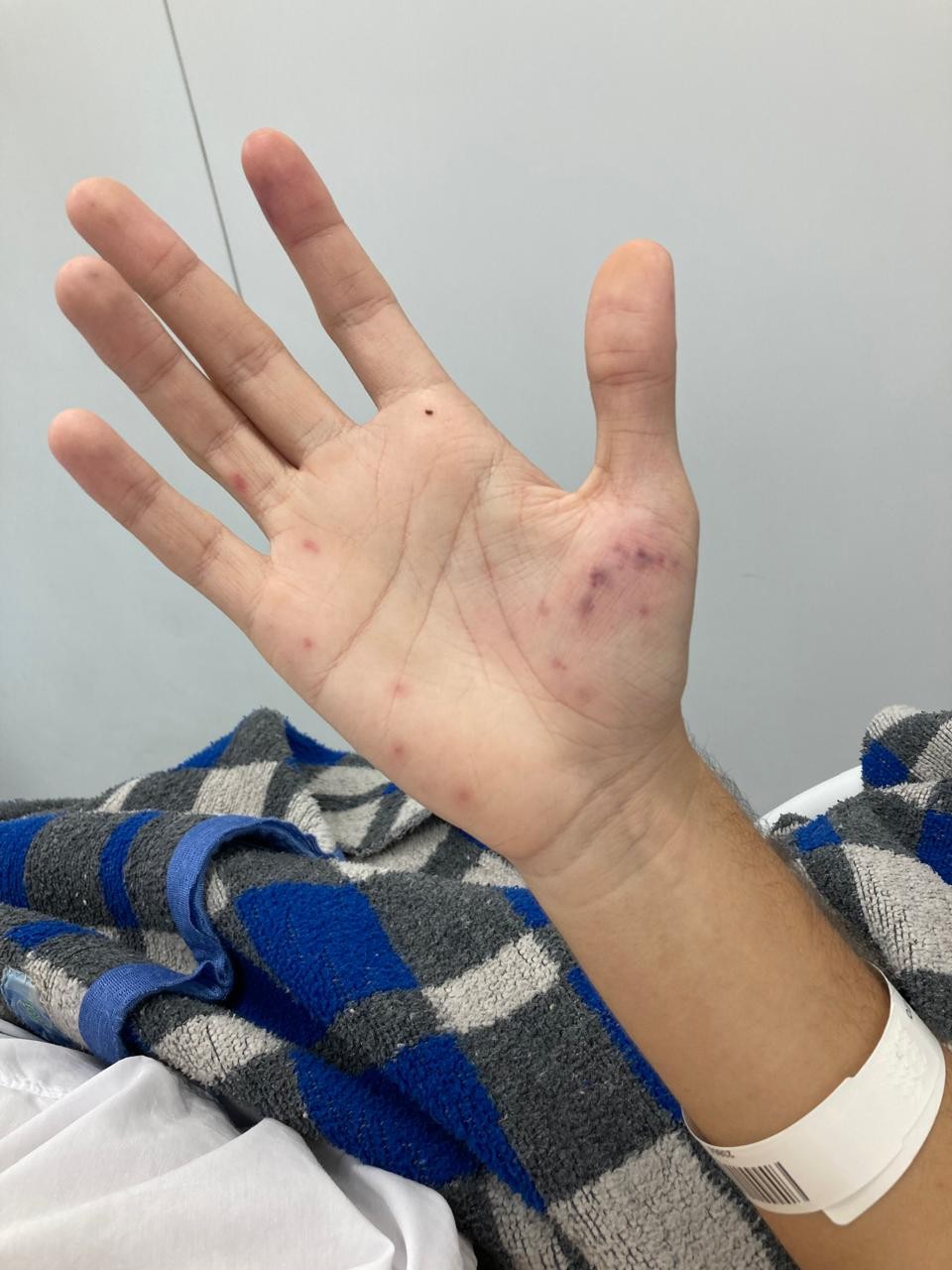
Figure 1. Petechial and purpuric rash on patient's right hand on day 1 of admission
A transthoracic echocardiogram, with a 3D reconstruction, on day 2 of hospitalization showed a large vegetation on the posterior leaflet of the mitral valve (MV) (Fig. 2 A,B). A transesophageal echocardiogram (TEE) on the 4th day of hospitalization revealed an enlarged left atrium, MV thickening of the posterior leaflet and an image on the atrial aspect measuring 19 x 10 mm, compatible with a vegetation. There were no signs of paravalvular abscess. The presence of a typical microorganism in two or more blood cultures associated with vegetation identified by imaging methods confirmed the diagnosis of definitive IE of the native MV by Staphylococcus aureus using the modified Duke criteria (6).
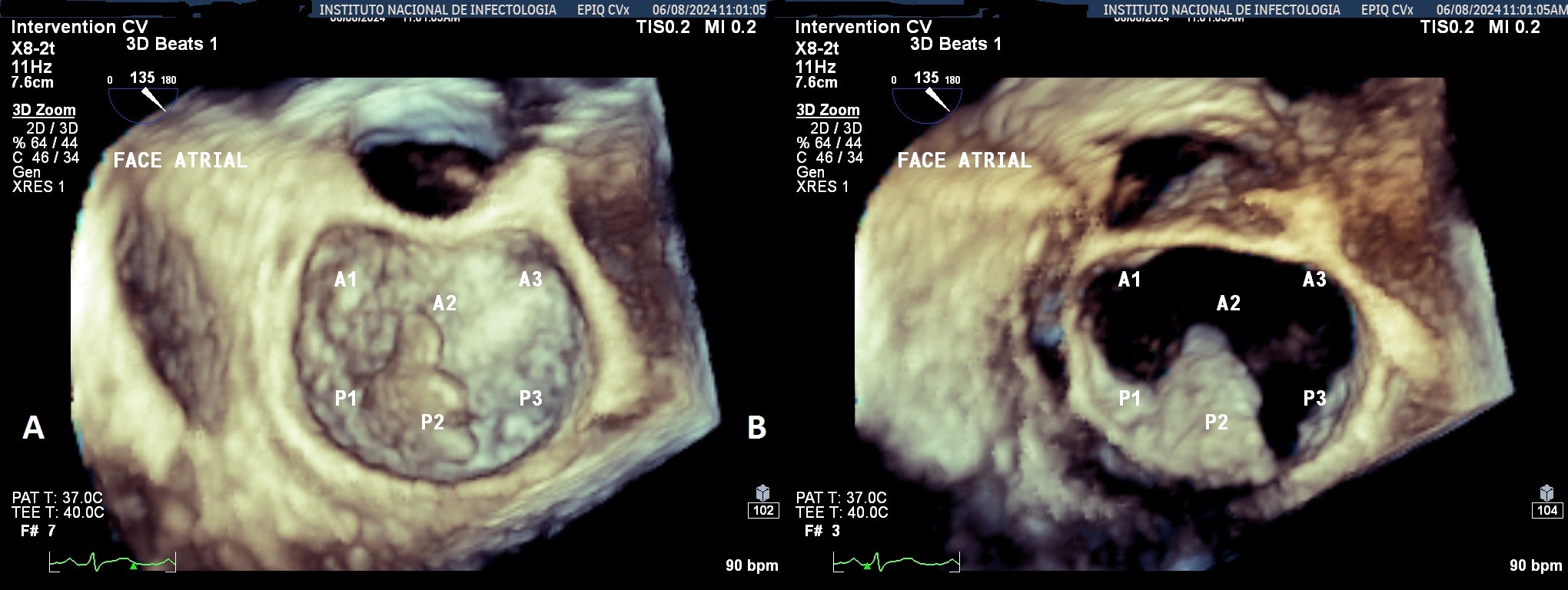
Figure 2. A) A 3D echocardiogram displaying a large vegetation on the atrial face of the mitral valve during the end of ventricular systole B) A 3D echocardiogram displaying the atrial face of the mitral valve during ventricular diastole, with a large vegetation
An abdominal CT scan with contrast on the 8th day of hospitalization (Fig. 3) showed parenchymal collections in the spleen, the largest measuring 10.8 x 10.5 x 7.1 cm, as well as a collection in the left renal cortex measuring 5.5 x 4.3 x 3.1 cm, secondary to septic embolism.
Figure 3. Computed tomography of the abdomen on the 8th day of hospitalization showing hypodense areas in the spleen and left renal cortex
He was transferred to a referral cardiac surgery hospital for surgical support, where he underwent splenectomy on the 13th day of hospitalization. The histopathology report of the splenic specimen revealed an enlarged spleen and multiple collections with a large amount of purulent material; the culture of the collection was positive for Staphylococcus aureus. On the third day after splenectomy, he maintained fever and control blood cultures were negative. He underwent a new TEE, which showed moderate MV regurgitation. On day 19, he maintained a fever and presented with pain in his right thigh, the distal pulses of the right lower limb were not palpable, but he maintained adequate perfusion, with no change in color. In view of the continuing fever and possible new embolic phenomena, rifampicin was added to the antimicrobial regimen (300mg q8h), angiotomography of the right lower limb was requested and the vascular surgery team was invited for consultation. The angiotomography of the right lower limb (Fig. 4) showed obstruction of the right tibiofibular trunk by 2.3 cm and of the proximal portion of the anterior tibial artery by 0.7 cm, with distal opacification of the vessels. The vascular surgery team advised starting IV unfractionated heparin, without the need for a surgical approach.
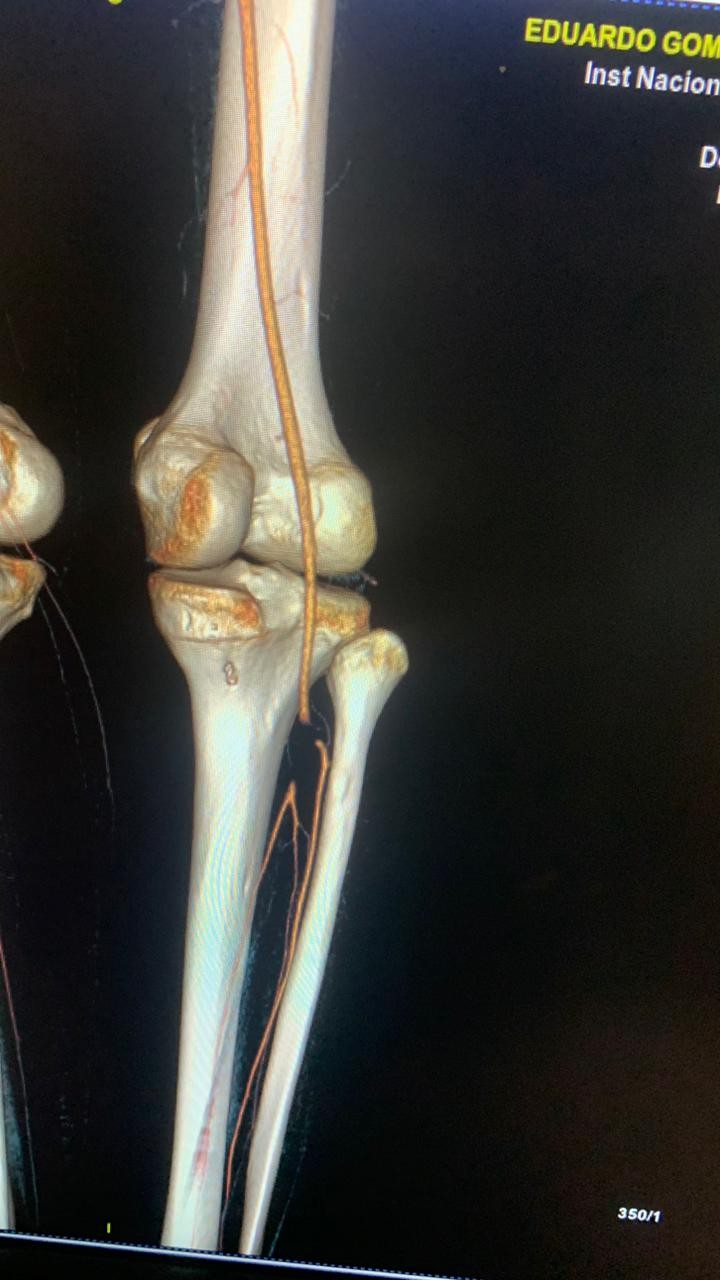
Figure 4. CT angiography of the right lower limb on 19th day of hospitalization showing an obstruction ( a “stop” ) of the right tibiofibular trunk
CT – computed tomography
Cardiac surgery was indicated due to persistent infection, fever and multiple embolic phenomena despite an adequate antimicrobial regimen on day 18. In the preoperative period, a magnetic resonance angiography of the brain was requested, which showed a recent lacunar infarction in the frontal region on the right and a parietal subarachnoid hemorrhage on the left. Despite the high risk of ischemia and loss of the lower limb in the event of cardiopulmonary bypass, MV repair with prosthetic ring placement was performed on day 22 of hospitalization. The surgical procedure was described as follows: The native MV showed an extensive vegetation across the entire surface of the posterior leaflet and a small area of the anterior leaflet. The vegetation was resected as was the affected segments of both leaflets; the posterior leaflet was reconstructed with an autologous pericardial patch and the anterior leaflet with surgical stitches. Separate Ethibond 2.0 stitches were placed around the MV ring and a number 32 annuloplasty ring was implanted.
Histopathology of the MV fragment showed degenerative changes (fibrosclerosis and myxoid degeneration) associated with the presence of adhered vegetation (adhered nodular area composed of fibrin and suppuration), Gram's stain showed gram-positive cocci, and the Grocott-Gomori stain was negative for fungi. He remained in the intensive care unit where he maintained a daily fever (38°C), but with a progressive drop in CRP. On day 29 of cardiac surgery, he presented a macular rash due to oxacillin, which was replaced by cefazolin. Control abdominal CT revealed regression of the hypocaptant renal lesion on the right and a small reduction in the volume of the abscess in the renal cortex on the left (Fig. 5). A CT scan of the brain was normal.
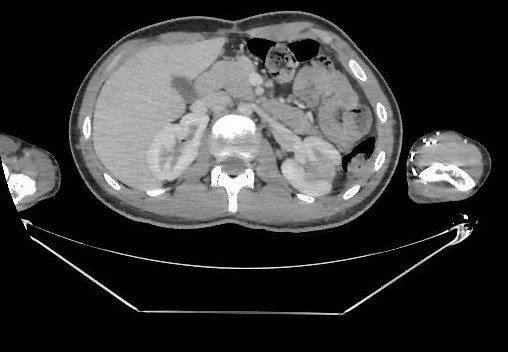
Figure 5. Computed tomography of the abdomen on the 27th day of hospitalization showing a decrease in the embolic lesion on the right kidney
On day 30 he was diagnosed with flu-like flu syndrome associated with rifampin, and the drug was discontinued. On day 48 he had an isolated febrile peak (38°C) with negative blood cultures, and a new abdominal CT scan revealed a small collection near the iliac muscle on the right. On day 54 he was discharged from hospital, asymptomatic and instructed to follow antimicrobial therapy with cotrimoxazole (800/160mg q12h) and anticoagulation with rivaroxaban 20mg q24h.
He returned for an outpatient appointment on day 62, having remained asymptomatic, undergone vaccination for encapsulated germs (S. pneumoniae, N. meningitidis, Haemophilus spp.) and was instructed to continue therapy with oral cotrimoxazole for a further two weeks (totaling eight weeks of antimicrobial therapy after heart surgery), and anticoagulation with rivaroxaban for 6 months.
The timeline of events is shown in Figure 6, for a quick overview.
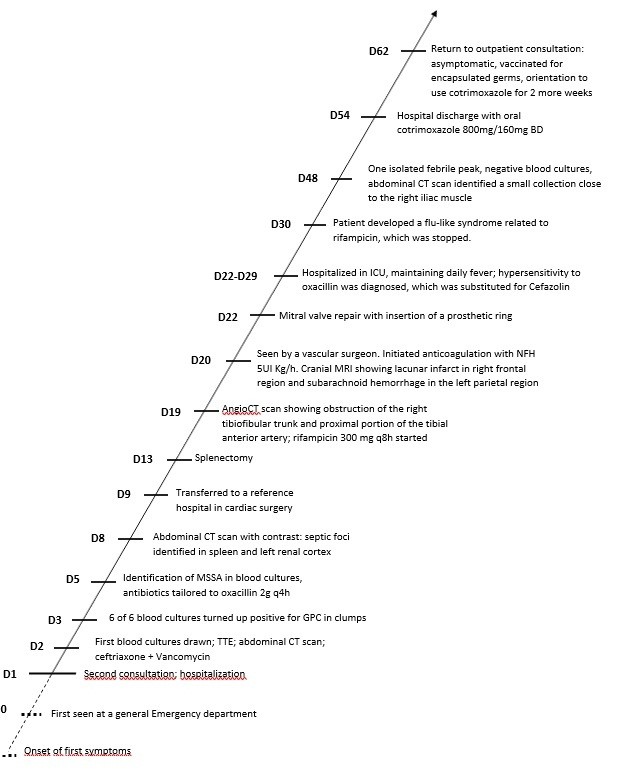
Figure 6. Timeline of events
TTE – transthoracic echocardiogram, CT – computed tomography, GPC –Gram-positive cocci, MSSA – methicillin-sensitive Staphylococcus aureus, UFH – unfractionated heparin, MRI-magnetic resonance imaging, ICU – intensive care unit
Discussion
We presented a case of an adolescent patient with definite community-acquired native valve IE due to Staphylococcus aureus according to the modified Duke criteria evolving with multiple embolic events, and with no identifiable portal of entry by clinical-epidemiological history.
This patient had no known previous valve disease, although histopathology identified myxomatous degeneration; although he had no clinically evident entry point for infection at the time of hospitalization, he had been exposed to multiple insect bites, which is why the hypothesis of dengue, in the context of a resident of Rio de Janeiro, was promptly raised.
In the pediatric population, the main risk factor for IE is congenital heart disease (CHD) (5, 7-9). However, there is a smaller proportion of cases in which there is no CHD, and no portal of entry had been identified, generally these episodes occur in late adolescents (from the age of 15), as well as in young adults and, in the case of the pediatric population, confers a worse prognosis to the episode (3, 5). Several studies point to an increase in the incidence of IE in the pediatric population in the scenario without association with heart disease (3-5). Staphylococcus aureus is the main pathogen observed in the pediatric population in developed countries in both European and American series. Day et al. (1) in a multicenter retrospective analysis during the years 2000 and 2003 analyzed 1588 cases of IE in the pediatric population, in which they observed a bimodal distribution with peaks in infancy (31 days to 1 year of age) and in late adolescence (17-20 years), and S. aureus was the main etiologic agent. Esposito et al. (10) in a retrospective multicenter study conducted in Italy from 2005 to 2015 found 47 pediatric patients with IE, of whom nine (19.1%) had no risk factors for IE, the main valve affected was the MV, and the main pathogen found in the group with CHD was oral/ viridans streptococci (37.9%) and in the group without risk factors, S. aureus (27.8%). The three deaths reported occurred in the group of patients without CHD (10). Marom et al. (4) in a retrospective single-center Israeli study from 1992 to 2004 analyzed 51 cases of IE in the pediatric population, of which 9 (18%) occurred in patients without CHD or any other risk factor for IE, all episodes were acquired in the community, but only in two was it possible to find the source of infection. In this group, all had left-sided valve involvement identified on echocardiography, and one had concomitant mitral and tricuspid valve involvement. S. aureus was the main etiologic agent identified, occurring in 33% of the episodes (4).
Despite the increased incidence of S.aureus infection associated with healthcare, community-acquired staphylococcal IE is a serious event, with significant morbidity even in previously healthy patients, as in the case presented (11, 12). The patient had no recent history of skin or soft tissue infection, nor any recent history of trauma or another obvious portal of entry. The differential diagnoses considered for the episode were dengue with alarm signs, since there were insect bites all over the body, but no signs of cutaneous infection. There was also an important epidemiological aspect, as he lived near a large local cemetery and reported many mosquitoes at home. On admission, he had an elevated CRP and a left shift, which suggested a bacterial infection; NS1, IgM and IgG tests for dengue were requested, all of which were negative. Although these negative laboratory tests for dengue do not rule out the diagnosis, this hypothesis was considered less likely. Disseminated gonococcal disease was also considered as a differential diagnosis, but there were no reports of compatible symptoms such as a history of polyarthralgia or tenosynovitis and there were no reports of risky sexual exposure. Another diagnosis that was considered was disseminated meningococcal disease without meningitis, as despite his vaccines being up to date, he worked with the public in a supermarket. However, the echocardiograms and CT scans, and the results of the subsequent blood cultures ruled out this hypothesis.
Cardiac surgery, with MV repair with placement of a prosthetic ring, was performed on the 22th day after the onset of the first symptoms. The indication for surgery was the presence of new embolic phenomena despite optimized antibiotic therapy. In this case, the patient had a large vegetation (19 x 10 mm) on the native MV caused by a microorganism with known emboligenic potential, which gave the episode a high risk of new embolic phenomena (13). The importance of early cardiac surgery in patients with native IE on left valves is already well established in the literature, with favorable results due to the reduction in embolic events after a surgical procedure performed within 1 week of the first symptoms (14). Surgery should not be postponed even in those with asymptomatic embolic events to the central nervous system (15). The choice of valve repair with placement of a prosthetic ring was due to the good outcomes of this technique when applied by a trained team, and especially because the patient was young, physically active, and would not need anticoagulation as he did not have a prosthesis inserted (16).
The consensus on surgical treatment in IE is to privilege repair whenever possible.
In our case, there was no significant valve regurgitation and a mitral ring was inserted. This means that this patient would not need anticoagulation for life, and nor would he have a bioprosthesis, which would mean a greater risk for further episodes of IE, and the need for a redo operation due to degeneration in the time span of around 10 years. In summary, he had the mitral ring inserted because he was a young man and his valve damage allowed for a more conservative surgical approach.
Regarding anticoagulation and its duration, the patient had rivaroxaban for 6 months because of the femoral artery occlusion, not because of the MV annuloplasty surgery. A direct oral anticoagulant (DOAC) was used, and not a vitamin K antagonist, because studies included in a recent meta-analysis have shown that warfarin use did not confer benefit in terms of thromboembolic prophylaxis after isolated MV repair in patients without atrial fibrillation or affect overall survival during the follow-up period of the included studies (17). Besides, the use of DOAC does not require monitoring, which was very important to this teenager, who had missed school and work during many weeks because of hospitalization.
This case also highlights the importance of carefully observing the evolution of patients, because even with adequate therapy, fever can persist, often in the context of distant abscesses, with the need to remove septic foci. It is therefore important to systematically screen for embolic phenomena in patients with IE. In this case, the pain in the left hypochondrium was initially interpreted as a warning sign of dengue fever, when a contrast-enhanced abdominal CT scan revealed a splenic abscess with significant involvement of the parenchyma, which led to splenectomy to remove the infectious focus. Fever can also be caused by hypersensitivity to drugs, which was observed in at least two episodes with the appearance of generalized pruritic macular exanthema after oxacillin infusion. In addition, the patient developed a flu-like syndrome due to rifampin, which was suspected due to fever and maintained leukocytosis, even with a recent change of the intravenous line and negative control blood cultures, which led to the drug being discontinued, and consequently the symptoms remitting.
Conclusions: This episode illustrates the emboligenic potential of Staphylococcus aureus, the importance of differential diagnosis with other diseases that present with fever, petechiae and purpura, with a special focus on epidemiological history to identify portals of entry for the etiological agent, and systematic screening for embolic phenomena in the clinical and surgical management of IE. The study also points to the need for careful follow up of patients treated to IE regarding antimicrobial adverse events.
Ethics: Informed consent was obtained from the subjects of the study. The local ethics committee reviewed and approved the study and assigned the approval number: CAAE: 87056925.3.0000.5272 on 25 mar. 2025
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: Conceptualization: NF, CL. Investigation: all authors. Writing - original draft preparation: NF, CL. Final writing review and editing: all authors. Thus, all authors fulfilled authorship criteria.
Acknowledgements and funding: We thank Fundação Pró Coração (Fundacor), Rio de Janeiro, for providing a grant to NF related to endocarditis research.
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Does not apply
References
| 1.Day MD, Gauvreau K, Shulman S, Newburger JW. Characteristics of children hospitalized with infective endocarditis. Circulation 2009; 119: 865-70. doi: 10.1161/CIRCULATIONAHA.108.798751 https://doi.org/10.1161/CIRCULATIONAHA.108.798751 PMid:19188508 |
||||
| 2.Gupta S, Sakhuja A, McGrath E, Asmar B. Trends, microbiology, and outcomes of infective endocarditis in children during 2000-2010 in the United States. Congenit Heart Dis 2017; 12: 196-201. doi: 10.1111/chd.12425 https://doi.org/10.1111/chd.12425 PMid:27885814 |
||||
| 3.Lin YT, Hsieh KS, Chen YS, Huang IF, Cheng MF. Infective endocarditis in children without underlying heart disease. J Microbiol Immunol Infect 2013; 46: 121-8. doi: 10.1016/j.jmii.2012.05.001 https://doi.org/10.1016/j.jmii.2012.05.001 PMid:22727890 |
||||
| 4.Marom D, Ashkenazi S, Samra Z, Birk E. Infective endocarditis in previously healthy children with structurally normal hearts. Pediatr Cardiol 2013; 34: 1415-21. doi: 10.1007/s00246-013-0665-9 https://doi.org/10.1007/s00246-013-0665-9 PMid:23483241 |
||||
| 5.Vicent L, Luna R, Martínez-Sellés M. Pediatric infective endocarditis: A literature review. J Clin Med 2022; 11: 3217. doi: 10.3390/jcm11113217 https://doi.org/10.3390/jcm11113217 PMid:35683606 PMCid:PMC9181776 |
||||
| 6.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633-8. doi: 10.1086/313753 https://doi.org/10.1086/313753 PMid:10770721 |
||||
| 7.Rosenthal LB, Feja KN, Levasseur SM, Alba LR, Gersony W, et al. The changing epidemiology of pediatric endocarditis at a children's hospital over seven decades. Pediatr Cardiol 2010; 31: 813-20. doi: 10.1007/s00246-010-9709-6 https://doi.org/10.1007/s00246-010-9709-6 PMid:20414646 PMCid:PMC2997359 |
||||
| 8.Pasquali SK, He X, Mohamad Z, McCrindle BW, Newburger JW, Li JS, et al. Trends in endocarditis hospitalizations at US children's hospitals: impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am Heart J 2012; 163: 894-9. doi: 10.1016/j.ahj.2012.03.002 https://doi.org/10.1016/j.ahj.2012.03.002 PMid:22607869 PMCid:PMC3408007 |
||||
| 9.Johnson JA, Boyce TG, Cetta F, Steckelberg JM, Johnson JN. Infective endocarditis in the pediatric patient: a 60-year single-institution review. Mayo Clin Proc 2012; 87: 629-35. doi: 10.1016/j.mayocp.2012.02.023 https://doi.org/10.1016/j.mayocp.2012.02.023 PMid:22766082 PMCid:PMC3497940 |
||||
| 10.Esposito S, Mayer A, Krzysztofiak A, Garazzino S, Lipreri R, Galli L, et al; Italian Pediatric Infective Endocarditis Registry. Infective Endocarditis in Children in Italy from 2000 to 2015. Expert Rev Anti Infect Ther 2016; 14: 353-8. doi: 10.1586/14787210.2016.1136787 https://doi.org/10.1586/14787210.2016.1136787 PMid:26708337 |
||||
| 11.Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. ICE Investigators. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293: 3012-21. doi: 10.1001/jama.293.24.3012 https://doi.org/10.1001/jama.293.24.3012 PMid:15972563 |
||||
| 12.Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, et al; AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis 2012; 54: 1230-9. doi: 10.1093/cid/cis199 https://doi.org/10.1093/cid/cis199 PMid:22492317 |
||||
| 13.Prendergast BD, Tornos P. Surgery for infective endocarditis: who and when? Circulation 2010; 121: 1141-52. doi: 10.1161/CIRCULATIONAHA.108.773598 https://doi.org/10.1161/CIRCULATIONAHA.108.773598 PMid:20212293 |
||||
| 14.Kim DH, Kang DH, Lee MZ, Yun SC, Kim YJ, Song JM, et al. Impact of early surgery on embolic events in patients with infective endocarditis. Circulation 2010; 122 (11 Suppl): S17-22. doi: 10.1161/CIRCULATIONAHA.109.927665 https://doi.org/10.1161/CIRCULATIONAHA.109.927665 |
||||
| 15.Fowler VG, Durack DT, Selton-Suty C, Athan E, Bayer AS, Chamis AL, et al. The 2023 Duke-International Society for Cardiovascular Infectious Diseases Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin Infect Dis 2023; 77: 518-26. doi: 10.1093/cid/ciad271 https://doi.org/10.1093/cid/ciad271 PMid:37138445 PMCid:PMC10681650 |
||||
| 16.Bonaros N, Czerny M, Pfausler B, Müller S, Bartel T, Thielmann M, et al. Infective endocarditis and neurologic events: indications and timing for surgical interventions. Eur Heart J Suppl; 22 (Suppl M): M19-M25. doi: 10.1093/eurheartj/suaa167 https://doi.org/10.1093/eurheartj/suaa167 PMid:33664636 PMCid:PMC7916418 |
||||
| 17.Papadimas, E., Tan, Y. K., Choong, A. M. T. L., Kofidis, T., & Teoh, K. L. K. (2021). Anticoagulation After Isolated Mitral Valve Repair: A Systematic Review and Meta-Analysis of Clinical Outcomes. Heart Lung & Circ 2020; 30: 247-53. https://doi.org/10.1016/j.hlc.2020.09.005 https://doi.org/10.1016/j.hlc.2020.09.005 PMid:33082110 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

