Predictors and outcomes of coronary ectasia presenting with acute coronary syndrome
ORIGINAL RESEARCH ARTICLE
Predictors and outcomes of coronary ectasia presenting with acute coronary syndrome
Article Summary
- DOI: 10.24969/hvt.2025.598
- CARDIOVASCULAR DISEASES
- Published: 15/09/2025
- Received: 02/08/2025
- Revised: 17/08/2025
- Accepted: 18/08/2025
- Views: 2349
- Downloads: 1291
- Keywords: : Acute coronary syndrome, aneurysm, angioplasty, anticoagulants, coronary artery disease, coronary artery ectasia
Address for Correspondence: Samir Rafla, Cardiology Department, Alexandria University, Faculty of Medicine, Alexandia, Egypt
Phone: +20 01001495577 E-mail: smrafla1@gmail.com
ORCID: Samir Rafla - 0000-0001-8688-6532; Elsayed Abdelaaty - 0009-0008-1162-9118; Amr Zaki - 0009-0001-6076-7225; Mohamed Sadaka - 0000-0001-6752-9337 Samir Rafla - 0000-0001-8688-6532
Elsayed Abdelaaty, Amr Zaki, Mohamed Sadaka, Samir Rafla
Cardiology Department, Alexandria University, Faculty of Medicine, Alexandia, Egypt
Abstract
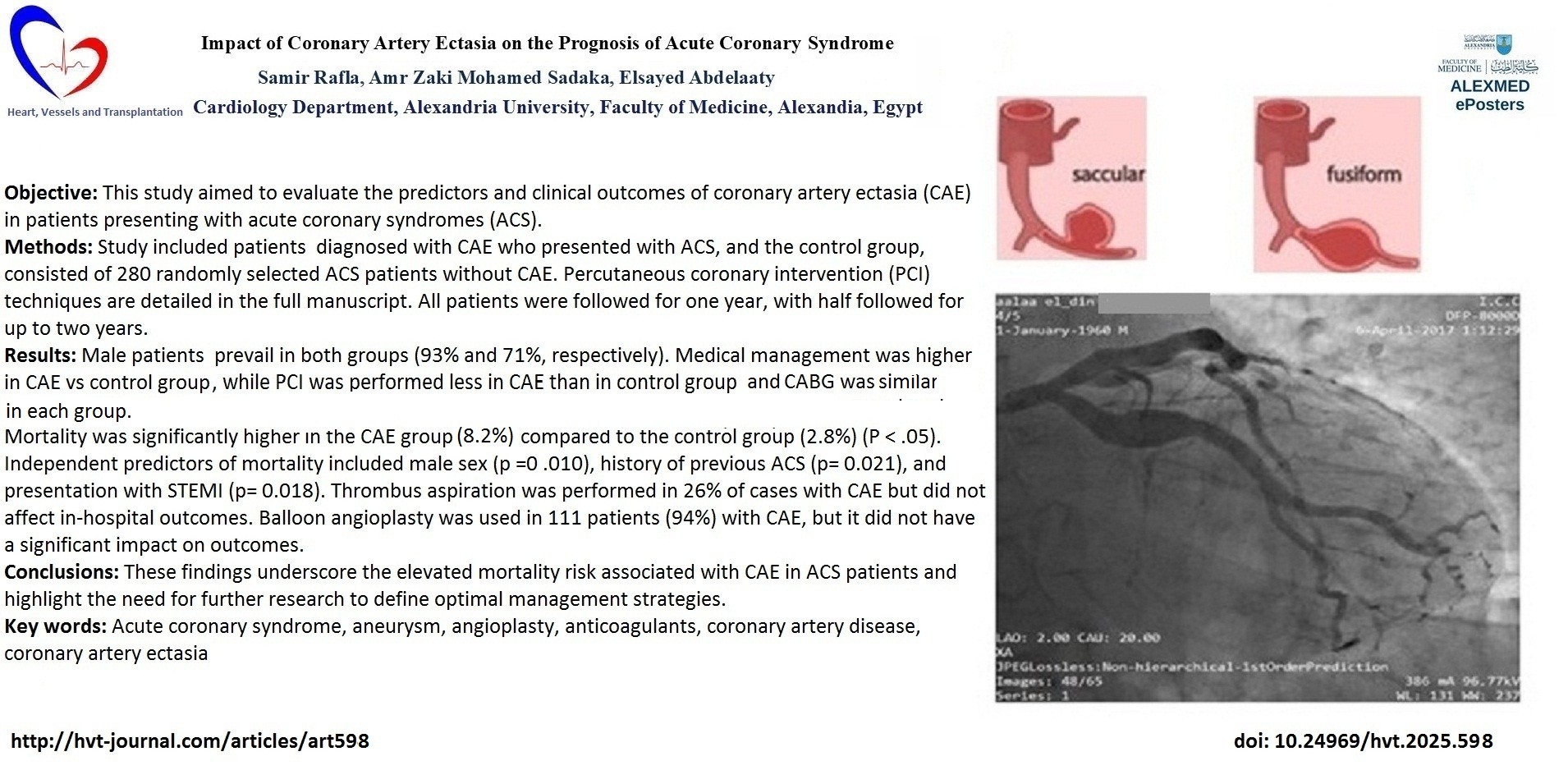
Objective: This study aimed to evaluate the predictors and clinical outcomes of coronary artery ectasia (CAE) in patients presenting with acute coronary syndromes (ACS).
Methods: Study included patients diagnosed with CAE who presented with ACS, and the control group, consisted of 280 randomly selected ACS patients without CAE. Percutaneous coronary intervention (PCI) techniques are detailed in the full manuscript. All patients were followed for one year, with half followed for up to two years.
Results: Male patients prevail in both groups (93% and 71%, respectively). Medical management was higher in CAE vs control group (34.6% vs 15%), while PCI was performed less in CAE than in control group (56.5% vs.77.8%) and coronary bypass surgery was performed in 8.9% and 7% of cases in each group. Mortality was significantly higher in the CAE group (8.2%) compared to the control group (2.8%) (p<0.05). Independent predictors of mortality included male sex (p=0.010), history of previous ACS (p=0.021), and presentation with ST-elevation myocardial infarction (p=0.018). Thrombus aspiration was performed in 26% of cases with CAE and balloon angioplasty was used in 111 patients (94%) with CAE, but both not have a significant impact on outcomes.
Conclusions: These findings underscore the elevated mortality risk associated with CAE in ACS patients and highlight the need for further research to define optimal management strategies.
Key words: Acute coronary syndrome, aneurysm, angioplasty, anticoagulants, coronary artery disease, coronary artery ectasia
Abbreviations:
ACS - acute coronary syndromes, CABG - coronary artery bypass grafting, CAD - coronary artery disease, CAE - coronary artery ectasia, CS - cardiogenic shock, LCX - left circumflex artery, LAD - eft anterior descending artery, MACE - major adverse cardiac event, MI - myocardial infarction, PCI - primary percutaneous coronary intervention, RCA - right coronary artery, STEMI - ST-segment elevation myocardial infarction, TVR - target vessel revascularization
Graphical abstract
Introduction
Coronary artery ectasia (CAE), or coronary artery aneurysm, is the aneurysmal dilatation of the coronary artery. A dilatation with a diameter of 1.5 times the adjacent normal coronary artery defines the diagnosis of ectasia (1-4). The prevalence varies between 1.2%-4.9%, with a male-to-female ratio of 3:1 (5-18). It is generally classified based on the shape and the extent of involvement of the coronary arteries. Classification based on shape is as following: a) the saccular-transverse diameter surpasses the longitudinal dimension; b) the diameter of the fusiform transverse is less than the longitudinal dimension.
Coronary ectasia is likely a heightened form of vascular remodeling. Proteolytic enzymes as cysteine proteinases participate in the pathogenesis of coronary ectasia (19). Hyperinsulinemia may increase the remodeling process in the setting of coronary atherosclerosis by stimulating the proliferation of vascular smooth muscle cells from the arterial media (19).
Coronary ectasia is diagnosed by coronary angiography. Intravascular ultrasound is valuable to assess luminal extension and vessel wall pathologies (19). Slow antegrade contrast filling and local contrast deposition in the dilated coronary segment (stasis) are signs of turbulent flow during angiography (20). The presence of aneurysmal segments produces slow blood flow. This is associated with an increased incidence of angina pectoris and myocardial infarction (21).
This study aimed to evaluate the predictors of coronary ectasia, as well as the in-hospital and intermediate-term outcomes for patients with ectatic coronary artery disease (CAD) presented with acute coronary syndromes (ACS).
Methods
Study design and population
This is a retrospective study and a prospective study.
All patients with ACS with ectatic CAD were studied retrospectively at the main university hospital and other centers from January 2016 until December 2018 and prospectively from January 2019 until June 2019. Demographic features, angiographic results, and clinical events were compared between ACS patients with and without CAE.
The ACS patients with ectatic CAD were assigned to group 1. All ACS patients who were catheterized and no ectasia was found were added to group 2.
There was no bias in selecting group II patients. Exclusion criteria: 1) patients with a previous history of coronary artery bypass graft surgery (CABG) or treated with emergent CABG; 2) patients with inconclusive clinical data from hospital files and computer records; 3) patients with cardiogenic shock.
All patients gave written informed consent to the study protocol. The study adhered to the principles outlined in the Declaration of Helsinki 2024. The protocol was approved with the Institution’s Ethics Committee's.
Baseline variables
All the patients with ectatic CAD were subjected to thorough history taking with special emphasis on: I. Clinical data of the patients:
1. Clinical presentation of acute coronary syndrome, either ST –elevation myocardial infarction (STEMI), non ST-elevation myocardial infarction (NSTEMI), or unstable angina;
2. Demographic features and clinical characteristics of the patients: age, sex, diabetes mellitus (insulin-dependent or non-insulin dependent), hypertension, smoking, dyslipidemia, family history of CAD, previous ACS, and previous coronary interventions;
3.Initial drug history and medications, including antiplatelets, anticoagulants, statins, beta-blockers, and others.
II. Standard 12-lead electrocardiogram.
III.Transthoracic echocardiography with special emphasis on left ventricular ejection fraction (EF), regional wall motion abnormalities (RWMA), and degree of mitral valve regurgitation.
Coronary angiography and percutaneous coronary intervention (PCI)
All patients underwent coronary angiography after informed consent forms were obtained. Angiography procedures were performed using the standard technique. Catheters were placed according to the indications in the guidelines. Angiograms were analyzed by two independent and experienced interventional cardiologists, and SYNTAX scores were calculated (20).
Diagnosis of ectatic coronary artery and classification was done according to the extent of involvement (types 1, 2, 3, and 4) and presence of aneurysm. Coronary ectasia was defined as the dilatation of a coronary segment with a diameter 1.5 times higher than normal adjacent segments. The decision about the ectatic coronary artery was made by visual assessment. We assessed number of ectatic vessels, TIMI flow before coronary intervention in ectatic and non-ectatic vessels. thrombus burden according to the TIMI thrombus scoring. The TIMI score 4 was defined as high-grade thrombus burden. After coronary angiography the decision of medical treatment, PCI or CABG was made and data recorded.
PCI procedures
PCI, thrombus aspiration and stenting with and without balloon predilatation were performed using standard techniques and approaches through radial or femoral access. We used guiding catheter 6F or 7F size and the guiding wire was either hydrophilic or non-hydrophilic. We collected the following data: single or multi-vessel PCI, thrombus aspiration, balloon predilatation, type of stents, either drug-eluting (DES) or covered stents (number of stents used, diameter, and length, use of intracoronary glycoprotein IIb/IIIa inhibitor, TIMI flow and myocardial blush grade post-procedure, PCI at the same setting as coronary angiography or staged PCI as regards the same vessel and time lapse in between.
Outcomes and follow-up
We collected data on In-hospital outcomes as death, MI, stroke, bleeding, arrhythmia, heart failure, target vessel revascularization (TVR), or non-TVR (major adverse cardiac events, MACE). Intermediate outcomes were collected with minimum follow-up of 6 months up to 3 years.
Data collection
All data was collected both retrospectively and prospectively. Demographic features and clinical characteristics of the patients with data regarding revascularization and recurrent events were obtained from hospital archives. Information about mortality for a year was obtained through the use of phone calls, national records, and the hospital records system. Hospital records were used to collect in-hospital outcomes, but long-term clinical data was collected by reviewing those records, conducting telephone interviews, and conducting outpatient visits.
Statistical analysis (22)
Statistical analysis was performed using the SAS System for Windows version 9.2 (SAS Institute Inc., Cary, NC). A computer database was used to enter data in a prospective manner. The study hypothesis was tested by assessing the association between ectasia and various risk factors or management techniques associated with it. To derive a list of other possible factors associated with ectasia formation, a range of clinical variables were also recorded prospectively, and exploratory comparisons were performed. A Fisher’s exact test and Chi-square test were used to compare categorical variables, while t test for independent samples or nonparametric Man Whitney test were used to compare continuous variables. Logistic regression analysis was used to define predictors of CAE and outcomes. The probability value < 0.05 was considered as statistically significant
Results
Clinical characteristics
As can be seen from Table 1, there were 280 patients with CAE in group 1 and 280 patients with ACS without CAE in group 2. Patients with CAE were predominantly male and smokers (both p<0.001), but had less often diabetes and dyslipidemia (p<0.05). They had a higher history of previous ACS (p=0.029) as compared to controls, and more often mitral regurgitation (p<0.001). The groups did not differ by presentation of ACS, history of PCI, ECG, RWMA or ejection fraction (p>0.05).
|
Table 1. Comparison of demographic and clinical data of the study population |
|||
|
Variables |
Group 1 (CAE) (n=280) |
Group 2 (Control) (n=280) |
p |
|
Male sex, % |
93 |
72 |
<0.001 |
|
Smoking, % |
57 |
40 |
<0.001 |
|
Hypertension, % |
61 |
65 |
NS |
|
Dyslipidemia, % |
21 |
28 |
0.05 |
|
Diabetes, % |
26 |
38 |
0.002 |
|
Renal impairment, % |
7.5 |
3.2% |
0.24 |
|
Previous ACS, n(%) |
22 (7.8) |
10 (3.5) |
0.029* |
|
Previous PCI, n(%) |
18 (6.4) |
79 (28) |
0.527 |
|
STEMI, n(%) |
79 (28) |
88 (31) |
0.406 |
|
NSTEMI, n(%) |
58 (21) |
65 (23) |
0.413 |
|
Unstable angina , n(%) |
143 (51) |
127 (46) |
0.176 |
|
Abnormal ECG, n(%) |
164 (58) |
151 (54) |
0.268 |
|
Mitral regurgitation, n(%) |
76 (27) |
40 (14) |
<0.001* |
|
RWMA, n(%) |
116 (41) |
115 (41) |
0.932 |
|
EF ≤35%, n |
10 |
8 |
NS |
|
EF>35, % (n (mean (SD)) |
270 (58.3(9.6)) |
272 (57(8.9)) |
0.087 |
|
*Statistically significant by Chi-square test, p<0.05 ACS - acute coronary syndrome, CAE – coronary artery ectasia, ECG – electrocardiogram, EF – ejection fraction, NSTEMI – non-ST-elevation myocardial infarction, PCI – percutaneous coronary intervention, RWMA – regional wall motion abnormalities, STEMI-ST-elevation myocardial infarction, |
|||
Management of ACS (Table 2)
Ninety seven cases (34.6%) were managed medically, but 43 (15%) in the control group, which is statistically
significant (p<0.001). PCI was done in 158 cases (56.5%) but in 218 of the control group (77.8%), (p<0.001). CABG was done in 25 cases (8.9%) and 19 patients of the control group (7%).
|
Table 2. Comparison of groups with or without CAE by management |
|||
|
Variables |
Group 1 (CAE) (n=280) |
Group 2 (Control) (n=280) |
p |
|
Medical, (%) |
97 (34.6) |
43 (15) |
p<0.001* |
|
PCI, n(%) |
158 (56.5) |
218 (77.8) |
p<0.001* |
|
CABG, n(%) |
25 (8.9) |
19 (7) |
0.868 |
|
Total, n |
280 |
280 |
|
|
p – Fischer exact test CABG – coronary bypass surgery, CAE – coronary artery ectasia, PCI – percutaneous coronary intervention |
|||
Data below are for 118 patients with ectatic CAD who presented with ACS and underwent PCI.
Most of the cases had a low Syntax score: 115 patients (97%) and 3 patients with an intermediate Syntax score (3%), with no statistically significant effect on hospital outcome among the 2 groups.
Regarding the anatomy of ectatic vessels (Fig. 1), right coronary artery (RCA) involvement was present in 70 patients (41%), left anterior descending artery (LAD) in 59 patients (35%), left circumflex artery (LCx) in 34 patients (20%), obtuse marginal (OM) in 3 patients, and left main disease in 2 patients, with no statistically significant effect on in-hospital outcome between different vessels.
Regarding the type of ectasia, type 1 was found in 22 patients (18%), type 2 in 14 patients (11%), type 3 in 32 patients (27%) and type 4 in 50 patients (42%). Aneurysm (Fig. 2) was found in 27 patients (23%); there was no statistically significant effect on in-hospital outcome between different types.
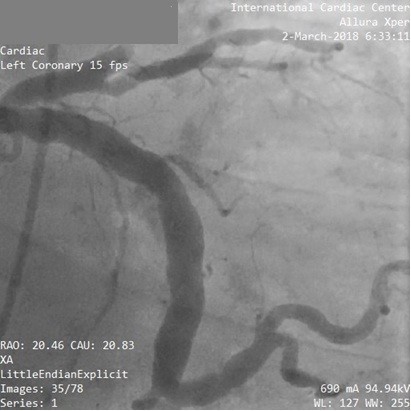
Figure 1. Coronary angiography showed diffuse ectasia of LCx and total occlusion of LAD in a 45-year-old male smoker presented with unstable angina. PCI was done to LAD with deployment of one DES
DES – drug-eluting stent, LAD – left anterior descending artery, LCx – left circumflex artery, PCI – percutaneous coronary intervention
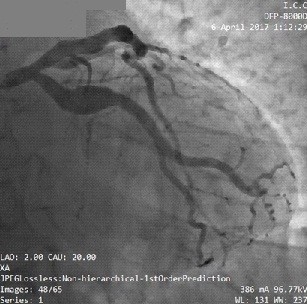
Figure 2. Coronary angiography shows an aneurysm of the left circumflex artery in a 52-year-old male patient with hypertension and a smoker presented with NSTEMI.
NSTEMI – non-ST –elevation myocardial infarction
TIMI flow before PCI was zero in 34 patients (29%), TIMI 1 in 10 patients (8%), TIMI 2 in 27 patients (23%), and TIMI 3 in 47 patients (40%), which were statistically significant effects on in-hospital bleeding (MCP = 0.011).
Thrombus aspiration was done to 31 patients (26%) with no statistically significant effect on hospital outcomes.
Most of the cases underwent balloon dilatation, 111 patients (94%) with no statistically significant effect on in-hospital outcome.
TIMI 0 flow post-PCI occurred only in one patient (0.8%), TIMI 1 in 2 patients (1.7%), TIMI 2 in 13 patients (11%) and TIMI 3 in 102 patients (86%) with no statistically significant effect on hospital outcomes.
Myocardial blush grade after PCI was zero in one patient (0.8%), grade 1 in 2 patients (1.7%), and grade 2 in 13 patients (11%), and grade 3 in 102 patients (86%) with no statistically significant effect on in-hospital outcomes.
A GPIIb/IIIa inhibitor was used in 45 patients (38%) with no statistically significant effect on hospital outcomes.
The TIMI score ranged from zero to 5, with a median of 0.5 and a statistically significant effect on an incidence of in-hospital bleeding (p =0.01).
Stent diameter ranged from 2.75 to 4.5 mm with a median of 4 mm, with a statistically significant effect on the incidence of in-hospital arrhythmia (p=0.03) and heart failure (p=0.02).
In hospital outcomes
In-hospital mortality has been witnessed in 3 ectatic patients (1.07%), but only in 2 patients of the control group (0.71%) Bleeding occurred in 19 patients of the ectatic group (6.78%) and 20 patients of the control group (7.14%).
Arrhythmia occurred in 19 patients of the first group (6.78%), but in 18 patients of the control group (6.42%).
Six patients of the ectatic group developed acute heart failure (2.14%) and 9 patients of the control group (3.21%).
TVR was indicated for one patient with coronary artery ectasia due to instant thrombosis, but not for any of the control groups.
Comparison of in-hospital outcomes in the ectatic group undergoing PCI (Tables 3, 4)
Most of the patients underwent stent deployment during PCI 102 (87%) but 14 patients underwent balloon dilatation only without stenting (13%) with no statistically significant effect on hospital outcome (Table 3).
NOACs was used in only 2 patients (1.7%) with no statistically significant effect on hospital outcome.
|
Table 3. Comparing patients with stents and patients without stents regarding hospital outcomes |
||||||||||||
|
Variables |
Death |
MI |
CVS |
Bleeding |
Arrhythmia |
HF |
||||||
|
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
|
|
No Stent Stent |
14 101 |
0 2 |
- |
- |
- |
- |
11 95 |
3 7 |
14 95 |
0 7 |
14 100 |
0 2 |
|
P |
1 |
- |
- |
0.1 |
0.5 |
1 |
||||||
|
p - Fisher’s exact significance CVS –cerebrovascular stroke , HF – heart failure, MI – myocardial infarction |
||||||||||||
|
Table 4. Hospital outcomes of patients on anticoagulants |
||||||||||||
|
Variables |
Death |
MI |
CVS |
Bleeding |
Non-TVR |
HF |
||||||
|
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
|
|
No Anticoagulant Anticoagulant |
114 2 |
2 0 |
- |
- |
105 2 |
10 0 |
108 2 |
7 0 |
113 2 |
2 0 |
||
|
p |
1 |
1 |
1 |
1 |
||||||||
|
p - Fisher’s exact significance CVS –cerebrovascular stroke, HF – heart failure, MI – myocardial infarction, TVR – target vessel revascularization |
||||||||||||
Comparison of intermediate outcomes in the ectatic group undergoing PCI (Tables 5-7).
There was a statistically significant effect on the incidence of MI as an intermediate outcome in patients who underwent balloon dilatation only without stenting (p=0.04) (Table 5.)
|
Table 5. Intermediate outcomes in patients with stents and without stents |
||||||||||||
|
|
Death |
MI |
CVS |
Bleeding |
Arrhythmia |
HF |
||||||
|
|
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
|
No Stent Stent |
13 91 |
1 10 |
11 91 |
2 1 |
12 92 |
1 0 |
- |
- |
- |
- |
13 90 |
0 2 |
|
p |
1 |
0.04* |
0.1 |
1 |
||||||||
|
p - Fisher’s exact significance CVS –cerebrovascular stroke, HF – heart failure, MI – myocardial infarction |
||||||||||||
Comparing patients on anticoagulants regarding intermediate outcomes showed NOAC use was associated an increased incidence of cardiovascular stroke in intermediate outcome (p=0.019).
|
Table 6. Comparison of intermediate outcomes of patients on anticoagulants |
||||||||||||
|
Variables |
Death |
MI |
CVS |
TVR |
Non TVR |
HF |
||||||
|
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
No |
Yes |
|
|
No Anticoagulant Anticoagulant |
102 2 |
12 0 |
101 1 |
2 1 |
103 1 |
0 1 |
100 2 |
3 0 |
101 2 |
2 0 |
101 2 |
2 0 |
|
p |
1 |
0.057 |
0.019* |
1 |
1 |
1 |
||||||
|
p - Fisher’s exact significance CVS –cerebrovascular stroke, HF – heart failure, MI – myocardial infarction, TVR – target vessel revascularization |
||||||||||||
Comparison of intermediate outcomes in all study population (Table 7)
Regarding follow-up of the cases, patients with ectatic CAD showed a higher mortality (23 patients, 8.2%) versus only 8 patients of the control group (2.8%), which is statistically significant (p=0.005).
|
Table 7. Intermediate outcomes in CAE and control groups |
|||
|
Variables |
Group 1 (CAE) (n=280) |
Group 2 (Control) (n=280) |
p |
|
Death, n(%) |
23(8.2) |
8(2.8) |
0.005* |
|
MI, n(%) |
7(2.5) |
3(1) |
0.171 |
|
CVS, n(%) |
1(0.4) |
0(0) |
0.48 |
|
Bleeding, n(%) |
1(0.4) |
0(0) |
0.303 |
|
Arrhythmia, n(%) |
0(0) |
2(0.8) |
0.499 |
|
HF, n(%) |
3(1) |
0(0) |
0.114 |
|
TVR, n(%) |
6(2.1) |
3(1) |
0.327 |
|
Non TVR, n(%) |
4(1.4) |
2(0.7) |
0.438 |
|
p - Fisher’s exact significance CVS –cerebrovascular stroke, HF – heart failure, MI – myocardial infarction, TVR – target vessel revascularization |
|||
Seven patients of the ectatic group developed MI (2.5%), and 3 patients of the control group (1%). Cardiovascular stroke occurred in one patient with ectatic CAD (0.35%), but not in the control group. Bleeding was encountered only in one patient of the first group (0.35%) but none of the control group.
None of the patients with ectatic CAD was found to have any arrhythmia (0%); on the other hand, arrhythmia occurred in 2 patients of the control group (0.8%). Three patients of the first group developed heart failure (1%) and none of the control group.
TVR was indicated to six patients with ectatic CAD (2.14%) and three patients in the control group (1%).
Non-TVR was indicated for 4 patients of the first group (1.4%) but only for two patients of the control group (0.7%).
Antiplatelets and anticoagulants of the study population (Table 8)
Clopidogrel was less often used as an antiplatelet in 193 patients with ectatic CAD (69%) and in 218 patients in the control group (77%) (p=0.024). Ticagrelor was prescribed as an antiplatelet drug in 80 ectatic patients (29%) but in 62 patients of the control group (22%). Warfarin was used in 5 patients of the ectatic group (1.7%) and 3 patients of the control group (1%). All without statistical significance. However, novel anticoagulants were used only in 4 (1.4%) patients in CAE group but none of the control group (p=0.061).
|
Table 8. Comparison of antiplatelets and anticoagulants in study groups |
|||
|
Variables |
Group 1 (CAE) (n=280) |
Group 2 (Control) (n=280) |
p |
|
Clopidogrel (Plavix), n(%) |
193(69) |
218(77) |
0.024* |
|
Ticagrelor (Brilique) , n(%) |
80(29) |
62(22) |
0.072 |
|
OAC, n(%) |
5(1.7) |
3(1) |
0.503 |
|
NOAC, n(%) |
4(1.4) |
0(0) |
0.061 |
|
p – *Chi-square test and Fisher's exact significance CAE – coronary artery ectasia, NOAC novel oral anticoagulant, OAC – oral anticoagulant |
|||
Predictors of adverse outcomes
Predictors of in-hospital mortality
In the multivariate logistic regression model, male sex (p=0.010), history of ACS (p=0.021), and presentation with STEMI (p=0.018) were found as independent predictors of in-hospital mortality (p =0.01).
Predictors of intermediate mortality
In the multivariate logistic regression model, the CAE (p=0.005) and presentation with STEMI (p=0.001) were found to be independent predictors of mortality as an indeterminate outcome.
Predictors of MACE
In a multivariate logistic regression model, stent diameter (p=0.005) and thrombus aspiration were significant predictors (p =0.047) of in-hospital MACE. The logistic regression model predicting intermediate outcome is not statistically significant (p =0.217)
Discussion
In our study, the conventional definition of CAE was applied. CAE prevalence was reported ranging from 0.9% to 5.3% (18). Yip et al. (15) reported CAE in the culprit vessel in 2.6% of 924 patients.
We found male sex, and smoking were associated with CAE, while diabetes and dyslipidemia were less often in our CAE patients. Coronary artery ectasia (CAE) patients with ACS was associated with significantly higher mortality (8.2% vs. 2.8%, p<0.05).
These factors have been linked to higher systemic inflammatory levels (19–21). Smoking, in particular, has been associated with coronary artery calcification (20), which may contribute to CAE pathogenesis.
In our study of male patients with CAE, a history of prior ACS was more common in the CAE group, potentially reflecting a higher burden of cardiovascular risk factors. Most patients with ACS and CAE presented with unstable angina, yet about half underwent PCI, the remainder received medical therapy or were referred for elective CABG.
Hospital MACE rates did not differ significantly between the CAE group and the control group. Independent predictors of in-hospital mortality were male sex, prior ACS, and STEMI presentation. For intermediate outcomes, both CAE and STEMI presentations emerged as independent predictors of mortality. A large thrombus burden was frequently observed in CAE patients, consistent with prior studies (14, 15).
Acute stent thrombosis rates were not significantly different between groups. Similarly, Karabulut et al. (23) found no difference in no-reflow rates between ectatic and non-ectatic arteries after primary PCI in STEMI patients. In our cohort, balloon angioplasty alone in 14 patients was associated with a higher rate of recurrent MI during follow-up, although the small sample size precludes firm conclusions on treatment strategy.
The RCA was most frequently involved in CAE, a pattern previously reported (12). Multivessel CAE was infrequent, occurring in approximately 25% of CAE cases (14). Most patients had low SYNTAX scores, which did not differ significantly between CAE and non-CAE groups; however, higher scores were associated with increased non-TVR events in intermediate outcomes, likely due to a greater prevalence of multivessel disease.
Zhang et al. (25), in a cohort of 512 CAE patients, found no significant difference in adverse cardiac events between low-grade and high-grade CAE (Markis classification), a finding mirrored in our study.
Regarding prognosis in patients with ectatic infarct-related artery, our in-hospital and long-term mortality rates were consistent with previous studies (23–31). Pre-PCI TIMI flow was 0 in 29% of cases, and aggressive antithrombotic therapy (including anticoagulants and GP IIb/IIIa inhibitors) was associated with an increased risk of in-hospital bleeding. Use of NOACs was linked to an intermediate outcome of cerebrovascular stroke in one patient, occurring in the setting of TIMI flow and MBG both being 0 post-PCI.
Study limitations
The study followed patients for one to two years; it did not delve into long-term complications such as recurrent MI, heart failure, or arrhythmias, which could offer a more complete picture of how CAE impacts long-term survival and quality of life.
Conclusions
Coronary artery ectasia in patients with ACS was associated with significantly higher mortality (8.2% vs. 2.8%, p<0.05).
The type of ectasia (true ectasia vs. aneurysm) also showed no significant difference in outcomes. Independent predictors of mortality included male sex, prior ACS, and STEMI presentation. Despite frequent use, thrombus aspiration and balloon angioplasty did not significantly improve outcomes. These findings highlight the need for further research into optimal management strategies for CAE in ACS to mitigate its elevated mortality risk.
Ethics: This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration 2024. The ethics department of our faculty of medicine approved the study protocol in 2019. Written informed consent was taken from all patients.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: S.A. data interpretation, manuscript writing, critical revision, A.Z. - idea and design of the work, responsible for coronary interventions, data analysis; M.S. - coronary interventions data analysis, interpretation E.A. – data collection, analysis and manuscript writing. All authors critically revised manuscript and approved for publication, thus fulfilled authorship criteria
Acknowledgements : We thank all residents and technicians who helped with the catheterization of patients
Funding: The study was done without any financial support (industry or grants)
Statement on A.I.-assisted technologies use: Authors declared they did not use AI-assisted technologies in reparation of this manuscript
Availability of data and materials: The master chart and patients’ files are available on request with E.A.
If data will be shared the rule of fair use apply with acknowledgement of authors and source or collaboration
References
| 1.Puymirat E, Simon T, Cayla G, Yves Cottin, Meyer Elbaz, Pierre Coste. et al. Acute myocardial infarction: changes in patient characteristics, management, and 6-month outcomes over a period of 20 years in the FAST-MI Program (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) 1995 to 2015. Circulation 2017; 136: 1908-19. doi: 10.1161/CIRCULATIONAHA.117.030798 https://doi.org/10.1161/CIRCULATIONAHA.117.030798 PMid:28844989 |
||||
| 2.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Circulation 2012; 126: 2020-35. doi: 10.1161/CIR.0b013e31826e1058 https://doi.org/10.1161/CIR.0b013e31826e1058 PMid:22923432 |
||||
| 3Panju AA, Hemmelgarn BR, Guyatt GH, Simel DL. The rational clinical examination. Is this patient having a myocardial infarction? JAMA 1998; 280: 1256-63. DOI: 10.1001/jama.280.14.1256 https://doi.org/10.1001/jama.280.14.1256 PMid:9786377 |
||||
| 4.Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, et al. Aneurysmal coronary artery disease. Circulation 1983; 67: 134-8. DOI: 10.1161/01.cir.67.1.134 https://doi.org/10.1161/01.CIR.67.1.134 PMid:6847792 |
||||
| 5.Hartnell GG, Parnell BM, Pridie RB. Coronary artery ectasia. Its prevalence and clinical significance in 4993 patients. Br Heart J 1985; 54: 392-5. DOI: 10.1136/hrt.54.4.392 https://doi.org/10.1136/hrt.54.4.392 PMid:4052280 PMCid:PMC481917 |
||||
| 6.Markis JE, Joffe CD, Cohn PF, Feen DJ, Herman MV, Gorlin R. Clinical significance of coronary arterial ectasia. Am J Cardiol 1976; 37: 217-22. DOI: 10.1016/0002-9149(76)90315-5 https://doi.org/10.1016/0002-9149(76)90315-5 PMid:1108631 |
||||
| 7Elahi MM, Dhannapuneni RV, Keal R. Giant left main coronary artery aneurysm with mitral regurgitation. Heart 2004; 90: 1430. DOI: 10.1136/hrt.2004.036293 https://doi.org/10.1136/hrt.2004.036293 PMid:15547021 PMCid:PMC1768590 |
||||
| 8.Kawsara A, Núñez GIJ, Alqahtani F, Moreland J, Charanjit RS, Alkhouli M. Management of coronary artery aneurysms. JACC Cardiovasc Interv 2018; 11: 1211-23. DOI: 10.1016/j.jcin.2018.02.041 https://doi.org/10.1016/j.jcin.2018.02.041 PMid:29976357 |
||||
| 9.Devabhaktuni S, Mercedes A, Diep J, Ahsan C. Coronary artery ectasia-a review of current literature. Curr Cardiol Rev 2016; 12: 318-23. DOI: 10.2174/1573403x12666160504100159 https://doi.org/10.2174/1573403X12666160504100159 PMid:27142049 PMCid:PMC5304254 |
||||
| 10.Roberts WC. Natural history, clinical consequences, and morphologic features of coronary arterial aneurysms in adults. Am J Cardiol 2011; 108: 814-21. DOI: 10.1016/j.amjcard.2011.05.009 https://doi.org/10.1016/j.amjcard.2011.05.009 PMid:21791334 |
||||
| 11.Antoniadis AP, Chatzizisis YS, Giannoglou GD. Pathogenetic mechanisms of coronary ectasia. Int J Cardiol 2008; 130: 335-43. DOI: 10.1016/j.ijcard.2008.05.071 https://doi.org/10.1016/j.ijcard.2008.05.071 PMid:18694609 |
||||
| 12Mavrogeni S. Coronary artery ectasia: from diagnosis to treatment. Hellenic J Cardiol 2010;51:158-63. PMID: 20378518 | ||||
| 13.Khedr A, Neupane B, Proskuriakova E, Jada K, Kakieu Djoss S, Mostafa JA. Pharmacologic management of coronary artery ectasia. Cureus 2021; 13: e17832. doi: 10.7759/cureus.17832C https://doi.org/10.7759/cureus.17832 |
||||
| 14. Ipek G, Gungor B, Karatas MB, Tonuk T, Keskin M, Tanik O, et al. Risk factors and outcomes in patients with ectatic infarct-related artery who underwent primary percutaneous coronary intervention after ST elevated myocardial infarction. Catheter Cardiovasc Interv 2016; 88: 748-53. DOI: 10.1002/ccd.26553 https://doi.org/10.1002/ccd.26553 PMid:27143640 |
||||
| 15.Yip HK, Chen MC, Wu CJ, Hang CL, Hsieh KYK, Fang CY, et al. Clinical features and outcome of coronary artery aneurysm in patients with acute myocardial infarction undergoing a primary percutaneous coronary intervention. Cardiology 2002; 98: 132-40. DOI: 10.1159/000066322 https://doi.org/10.1159/000066322 PMid:12417812 |
||||
| 16.Krüger D, Stierle U, Herrmann G, Simon R, Sheikhzadeh A. Exercise-induced myocardial ischemia in isolated coronary artery ectasias and aneurysms ("dilated coronopathy"). J Am Coll Cardiol 1999; 34: 1461-70. DOI: 10.1016/s0735-1097(99)00375-7 https://doi.org/10.1016/S0735-1097(99)00375-7 PMid:10551693 |
||||
| 17. Demopoulos VP, Olympios CD, Fakiolas CN, Pissimissis EG, Economides NM, Adamopoulou E. et al. The natural history of aneurysmal coronary artery disease. Heart 1997; 78: 136-41. 84. DOI: 10.1136/hrt.78.2.136 https://doi.org/10.1136/hrt.78.2.136 PMid:9326986 PMCid:PMC484892 |
||||
| 18. Giannoglou GD, Antoniadis AP, Chatzizisis YS, Damvopoulou E, Parcharidis GE, Louridas GE. Prevalence of ectasia in human coronary arteries in patients in northern Greece referred for coronary angiography. Am J Cardiol 2006 ;98: 314-8. DOI: 10.1016/j.amjcard.2006.02.034 https://doi.org/10.1016/j.amjcard.2006.02.034 PMid:16860015 |
||||
| 19.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension 2011; 57: 13240. DOI: 10.1161/HYPERTENSIONAHA.110.163576 https://doi.org/10.1161/HYPERTENSIONAHA.110.163576 PMid:21149826 PMCid:PMC3028593 |
||||
| 20. Head SJ, Farooq V, Serruys PW, Kappetein AP. The SYNTAX score and its clinical implications. Heart 2014; 100:1 69-77. Doi: 10.1136/heartjnl-2012-302482 https://doi.org/10.1136/heartjnl-2012-302482 PMid:23539552 |
||||
| 21. Papadakis MC, Manginas A, Cotileas P, Demopoulos V, Voudris V, Pavlides G, et al. Documentation of slow coronary flow by the TIMI frame count in patients with coronary ectasia. Am J Cardiol 2001; 88: 1030-2. doi: D10.1016/s0002-9149(01)01984-1 https://doi.org/10.1016/S0002-9149(01)01984-1 PMid:11704003 |
||||
| 22. Zulfiqar Ali, S Bala Bhaskar. Basic statistical tools in research and data analysis. Indian J Anaesth 2016; 60: 662-9. doi: 10.4103/0019-5049.190623 https://doi.org/10.4103/0019-5049.190623 PMid:27729694 PMCid:PMC5037948 |
||||
| 23. McEvoy JW, Blaha MJ, DeFilippis AP, Lima JAC, Bluemke DA, Hundley WG, et al. Cigarette smoking and cardiovascular events: role of inflammation and subclinical atherosclerosis from the MultiEthnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015; 35: 700-9. https://doi.org/10.1161/ATVBAHA.114.304562 https://doi.org/10.1161/ATVBAHA.114.304960 |
||||
| 24.Pauletto P, Rattazzi M. Inflammation and hypertension: the search for a link. Nephrol Dial Transplant 2006; 21: 850-3. DOI: 10.1093/ndt/gfl019 https://doi.org/10.1093/ndt/gfl019 PMid:16464884 |
||||
| 25.Zhang Y, Huang QJ, Li XL, Guo YL, Zhu CG, Wang XW, et al. Prognostic value of coronary artery stenoses, Markis class, and ectasia ratio in patients with coronary artery ectasia. Cardiology 2015; 131: 251-9. DOI: 10.1159/000381702 https://doi.org/10.1159/000381702 PMid:25997533 |
||||
| 26.Karabulut A, Cakmak M, Uzunlar B. Association between preinfarction angina and coronary artery ectasia in the acute myocardial infarction. Acta Cardiol 2011; 66: 509-14. DOI: 10.1080/ac.66.4.2126601 https://doi.org/10.1080/AC.66.4.2126601 PMid:21894809 |
||||
| 27. Harikrishnan S, Sunder KR, Tharakan J, Titus T, Bhat A, Sivasankaran S, et al. Coronary artery ectasia: angiographic, clinical profile and follow-up. Indian Heart J 2000; 52: 547-53. | ||||
| 28.Gunasekaran P, Stanojevic D, Drees T, Fritzlen J, Haghnegahdar M, McCullough M, et al. Prognostic significance, angiographic characteristics and impact of antithrombotic and anticoagulant therapy on outcomes in high versus low-grade coronary artery ectasia: A long-term follow-up study. Catheter Cardiovasc Interv 2019; 93: 1219-27. DOI: 10.1002/ccd.27929 https://doi.org/10.1002/ccd.27929 PMid:30393992 |
||||
| 29.Furugen M, Takagawa Y. Staged interventional management of a massive thrombus related to coronary artery ectasia in acute coronary syndrome. Cardiovasc Interv Ther 2012; 27: 57-61. DOI: 10.1007/s12928-011-0083-y https://doi.org/10.1007/s12928-011-0083-y PMid:24122644 |
||||
| 30. Devabhaktuni S, Mercedes A, Diep J, Ahsan C. Coronary artery ectasia- A review of current literature. Curr Cardiol Rev 2016; 12: 318-23. doi: 10.2174/1573403x12666160504100159 https://doi.org/10.2174/1573403X12666160504100159 PMid:27142049 PMCid:PMC5304254 |
||||
| 31.Fujii T, Sakai K, Kimura M, Nakano M, Ohno Y , Nakazawa G, et al. Coronary flow improvement following unsuccessful primary percutaneous coronary intervention in ST-elevation myocardial infarction with diffuse ectatic coronary artery. Eur Heart J Acute Cardiovasc Care 2017; 6: 623-31. DOI: 10.1177/2048872616633850 https://doi.org/10.1177/2048872616633850 PMid:26880852 |
||||
| 32. Takahito D, Kataoka Y, Noguchi T, Shibata T, Nakashima T, Kawakami S, et al. Coronary artery ectasia predicts future cardiac events in patients with acute myocardial infarction. Arterioscler Thromb Vasc Biol 2017; 37: 2350-5. 10.1161/ATVBAHA.117.309683 https://doi.org/10.1161/ATVBAHA.117.309683 PMid:29051141 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

