From Editor-in-Chief: On our performance, current issue, news from AHA 2025, guidelines and welcome to a new Editor
EDITORIALS
From Editor-in-Chief: On our performance, current issue, news from AHA 2025, guidelines and welcome to a new Editor
Article Summary
- DOI: 10.24969/hvt.2025.615
- CARDIOVASCULAR DISEASES
- Published: 11/12/2025
- Received: 09/12/2025
- Accepted: 09/12/2025
- Views: 1173
- Downloads: 604
- Keywords: Biomedical publishing, guidelines, trials, cardiovascular medicine, surgery, transplantation, internal medicine, public health
Address for Correspondence: Gulmira Kudaiberdieva, Editor-in-Chief, Heart, Vessels and Transplantation
E-mail: editor@hvt-journal.com

From Editor-in-Chief: On our performance, current issue, news from AHA 2025, guidelines and welcome to a new Editor
Graphical abstract
Key words: Biomedical publishing, guidelines, trials, cardiovascular medicine, surgery, transplantation, internal medicine, public health
Dear readers,
First, we wish you happy New Year holiday season, health, peace everywhere and all success.
Concluding Year 2025, I would like to start with updates: we applied to PMC NLM in September 2025 (under evaluation) and our evaluation by Clarivate for inclusion in their databases is ongoing.
We also happy to share that number of citations in SCOPUS database increased (156) and 1/4th of our articles received citations, our Hirsch index is 4 (Fig. 1). As you can see, our CiteScore for 2025 is 0.3 as compared to 0.2 in 2024. This is a good news, despite increase of articles published, our number of citations increased as well. We aim to improve further our indicators, increase quality of content and its presentation.
In current December 2025 issue of the journal, you can find 3 Editorials introducing what is new in recent ESC 2025 guidelines on management of cardiovascular diseases (CVD) and pregnancy, CVD and mental health, and myocarditis and pericarditis prepared by our invited experts and Editor. Among original research articles including meta-analysis in the field of cardiac surgery, you may find the articles on prognostic biochemical and genetic markers in patients with congenital heart defects undergoing cardiac surgery; study on comparison of echocardiographic parameters in evaluation of outcomes of different techniques of tricuspid valve repair with mitral valve replacement; and meta- analysis on effects of different mitral valve repair techniques on left ventricular function and article on frailty index as predictor of transcatheter aortic valve replacement (TAVR) outcomes in elderly patients. In the field of cardiology, we present experimental study on the effect of progesterone on cardiomyocytes in traumatic brain injury; effects of smoking on heart function;, economic analysis of cardiology drugs prescription; case series on left ventricular dysfunction and Takotsubo cardiomyopathy; neurovascular diseases – predictors of stroke-related pneumonia development; general surgery and oncology– surgery for the malignant tumors of biliopancreatodudodenal region. You can also read review article on sudden cardiac death in arrhythmogenic syndromes; and three interesting rare case reports: on osteomyelitis with hematogenic dissemination; Chagas disease presented as arrhythmia storm and implantation of leadless pacemaker in a patient with small heart – a new approach.
AHA 2025 sessions took place in November 7-10, 2025 and several interesting trials attract attention. Among them trials on arrhythmia as DARE-AF, VANISH 2, and DECAF (2-4):
-DARE – AF (2) was a randomized trial to assess the effect of sodium glucose co -transporter (SGLT) -2 inhibitor dapagliflozin on atrial fibrillation (AF) recurrence in patients without special indications like diabetes, heart failure or chronic kidney disease who underwent catheter ablation for persistent AF. Authors did not find differences between groups with or without dapagliflozin in AF burden or AF recurrence after 3 months treatment. Randomized controlled trials (RCT) should be undertaken for patients with special indications for SGLT-2 inhibitor to confirm observations that it reduces AF recurrence.
-The VANISH 2 study (3) demonstrated that catheter ablation of ventricular tachycardia (VT) (VT storm, appropriate ICD shock or antitachycardia pacing or sustained VT terminated at emergency department) in patients after myocardial infarction(MI) with ICD as compared to antiarrhythmic therapy (sotalol or amiodarone) reduced composite event (death from any cause, VT storm, appropriate ICD shock or sustained VT treated by medical intervention) by 25% (HR 0.75 95%CI 0.58-0.97, p=0.03). Good results of curative therapy that might improve the quality of life of patients and avoid side effects of drug therapy. Timing of ablation after MI is of interest.
-Another interesting DECAF trial demonstrated that at least one cup of coffee consumption per day had a protective effect against recurrence of AF (4). Two hundred patients, who underwent cardioversion for persistent AF, were randomized into 2 groups: 47% for caffeinated coffee consumption and 61% - abstinence group. During follow-up of 6 months AF/ atrial flutter recurrence was lower by 39% in caffeine group as compared to abstinence group (HR 0.61, 95% CI 0.42-0.89, p=0.01).
Among surgery trials, CAVIAR RCT trial tested whether proprotein convertase subtilisine /kexin 9 (PCSK9) inhibitor alirocumab in addition to rosuvastatin reduces the allograft vasculopathy after heart transplantation. Overall 57 patients who underwent heart transplantation received alirocumab and 57 were prescribed placebo. Alirocumab did not affect plaque volume 1 year after follow-up, nor fractional flow reserve, coronary flow reserve, or index of microcirculation but significantly reduced low-density lipoprotein (LDL) cholesterol levels as compared to placebo (5).![]()
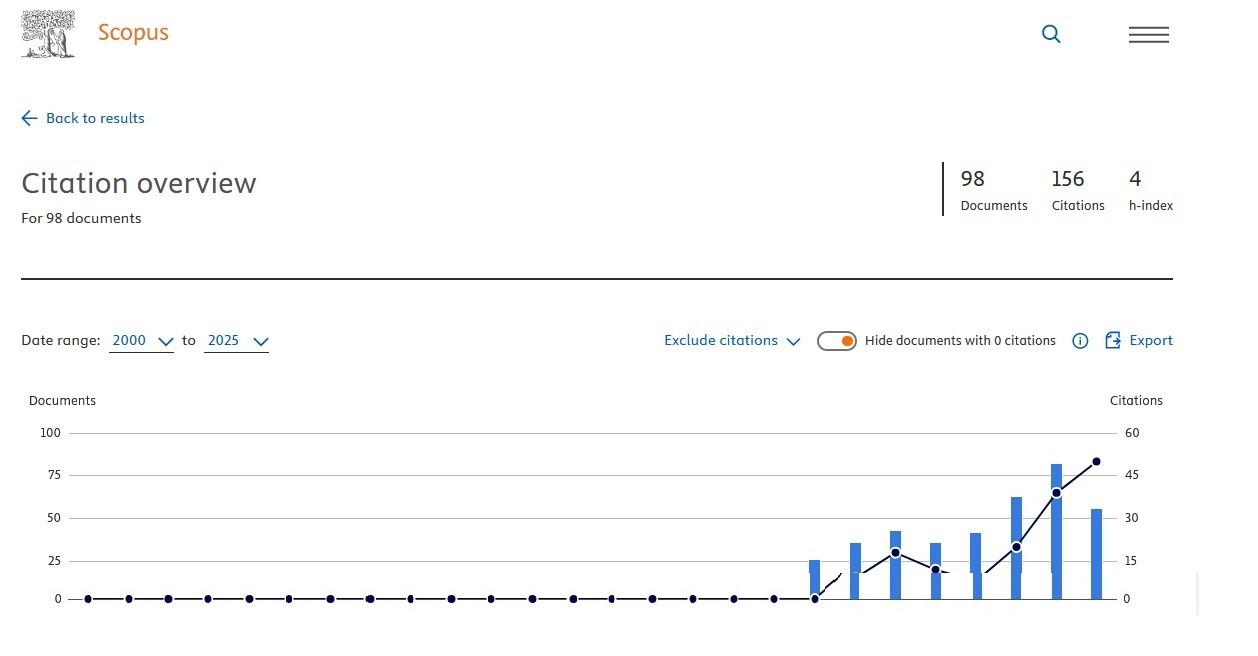
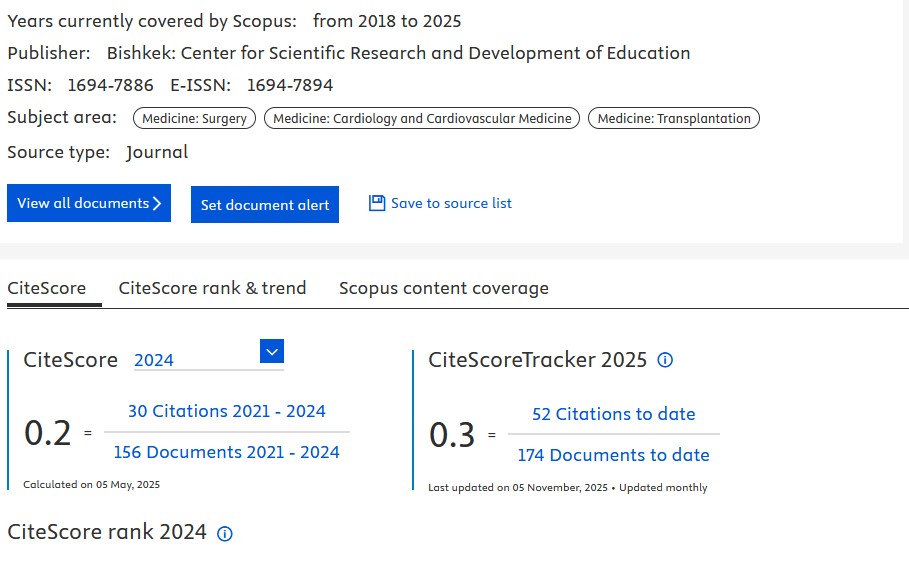
Figure 1. SCOPUS Hirsch index, number of citations and cited documents (upper panel) and SCOPUS CiteScore (bottom panel) panel by December 2025 for Heart, Vessels and Transplantation (1)
Heart, Vessels and Transplantation 2025; 9: doi: 10.24969/hvt.2025.615
From Editor-in-Chief Kudaiberdieva
![]()
In another trial, investigators studied whether hyperoxia during cardiopulmonary bypass (CPB) is associated with mortality or major adverse cardiac events (MACE) as cardiopulmonary resuscitation, extracorporeal membrane oxygenation, stroke and mortality in neonates undergoing cardiac surgery across 22 centers. They reported that PaO2 > 221mmHG is associated with mortality and MACE after adjustment for center the association weakened but remained for babies undergoing surgery for single ventricle. It means that monitoring for hyperoxia in patients with single ventricle anatomy should be performed during CPB (6).
ALIGN-AR trial, a new study on use of Trilogy for transcatether valve intervention for symptomatic moderate-to severe and severe aortic regurgitation (AR) with high surgical risk was conducted in 30 centers in USA. The 30-day MACE included death, major life-threating bleeding, acute kidney injury, major vascular complications, need for surgery or percutaneous coronary intervention (PCI), new pacemaker implantation and moderate and severe AR at high surgical risk. The trial showed the safety and effectiveness of dedicated valve as performance goals were reached. Technical success was 95%, 30-day primary endpoint occurred in 24% (performance goal reached). Post-procedure there were reduced AR, favorable hemodynamics, myocardial remodeling, improvement of quality of life, and functional status (7). Thus there is promising therapy for patients with severe AR at high surgical risk.
The VESALIUS –CV trial (8) demonstrated that evolocumab treatment as compared to placebo on optimal lipid lowering therapy in patients with atherosclerosis or diabetes without previous MI or stroke reduced by 25% MACE (death from coronary artery diseases, MI, ischemic stroke) and 4 -point MACE (all above plus ischemia-driven revascularization) by 19%. The mean LDL on PCSK9 inhibitor was 45 mg/dl and placebo 109 mg/dl. This shows that to reach target LDL in patients with atherosclerosis that cannot be done on other therapies use of evolocumab is beneficial, as it reduces MACE.
BROAD trial on intensive blood pressure therapy with a goal of 120/80 mm Hg as compared to standard in patients with diabetes type 2 demonstrated that intensive therapy reduced adverse cardiovascular (CV) events by 21% (HR 0.79, 95%CI 0.69-0.90, p<0.001) during 4.2 years of follow-up. Targeting lower levels of blood pressure in patients with type 2 diabetes and CV risk is associated with reduction of MACE development (9).
In CELEBRATE trial (10), novel antiplatelet zalunfiban injection at first medical contact reduced adverse events in patients with MI undergoing PCI by 21% (OR-0.79, 95%CI 0.65-0.98, p=0.028). There were higher infarct-related artery (IRA) patency and reduced 30-day all- cause death, stroke, recurrent MI, stent thrombosis and heart failure in zalunfiban group. Thus, we have effective antiplatelet treatment to be used at early stage of MI before PCI to facilitate IRA patency restoration and reduce unfavorable events.
Finally, high dose influenza vaccine (FLUNITY HD trial, 11) is found to markedly decrease hospitalizations for CVD, respiratory diseases and heart failure in analysis of 466 320 older adults among them ¼ had CVD.
I would like to briefly mention here two new guidelines: one is ESC 2025 update of lipid guidelines (12) and new WHO guidelines on use glucagon-like peptide (GLP1) receptor agonists in patients with obesity and diabetes (13, 14).
2025 Update of ESC lipid guidelines (12) focused on several changes: 1) the new 10-year risk stratification scores 2) new LDL lowering therapy – bempedoic acid and evinacumab, especially for patients with homozygous familial hypercholesterolemia; 3) new recommendations for lipid lowering therapy during hospitalization for acute coronary syndrome (ACS) 4) recommendation on lipoprotein (a); 5) recommendations for treatment of hypertriglyceridemia and 6) recommendations for preventions of CVD in patient HIV and cancer at very high risk of chemotherapy –related CV toxicity and 7) dietary supplements.
SCORE 2 is recommended for estimation of fatal and nonfatal 10-year CV risk in apparently healthy subjects <70 years without established atherosclerotic CVD, diabetes, chronic kidney disease, genetic/rare lipid or blood pressure disorders, while SCORE 2-OP is recommended for people ≥70 years old with same characteristics as above. You may find the pharmacological primary prevention initiation in patients with different risk and LDL levels when nonpharmacological measures are ineffective. Also authors made easily comprehend LDL treatment goals across population different total CV risk.
Non-statin therapies are recommended for patients who are intolerant to statins or who are at very high and high risk and cannot achieve LDL goal.
Bempedoic acid is recommended to patients who are unable to take statin therapy, its addition to the maximally tolerated dose of statin with or without ezitimibe should be considered in patients at very high and high risk to meet LDL goal.
Evinacumab, a mAb against angiopoietin like 3, should be considered in a patient older 5 years with homozygous familial hypercholesterolemia who are non at LDL- C goal despite maximal doses of lipid-lowering therapy.
Intensive lipid lowering therapy is recommended in ACS patients to reduce LDL level and improve outcomes. Intensification of lipid lowering therapy during ACS hospitalization is recommended for patients who were on lipid lowering therapy before hospitalizations.
Combination therapy with high intensity statin plus ezetimibe during ACS hospitalizations should be initiated in lipid lowering therapy naïve patients who are not expected to achieve LDL goal with statin therapy alone.
Finally Lp(a) more than 50 mg/dl (105 nmol/l) should be considered as CV risk-enhancing factor.
In December 2025, WHO recommended GLP1- agonists for treatment of obesity defined as body mass index (BMI) >30kg/m2 (13). WHO recommends (13) that GLP1 therapy may be used for adults living with obesity as long-term treatment. Patients living with obesity should receive counseling on behavioral and lifestyle changes, including physical activity and healthy diet. In patients receiving GLP1 agonists and GLP1/glucose-dependent insulinotropic polypeptide dual receptor agonist, behavioral and lifestyle counseling should be provided as first step to amplify the optimal health outcomes. In adults, living with obesity and prescribed GLP1 agonists, behavioral therapy should be part of multimodal clinical treatment algorithm.
In September 2025, WHO added GLP1 receptor agonists to the essential medicines list for treatment of diabetes type 2 with heart or kidney disease to improve blood glucose levels, support weight control, reduce risk of heart and kidney complications, and death (14). GLP1 receptor agonists – semaglutide, dulaglutide and liraglutide and GLP1/glucose-dependent insulinotropic polypeptide dual receptor agonist (trizepatide) are indicated for patients with type 2 diabetes, with established CVD or chronic kidney disease and obesity (BMI ≥30 kg/m2).
We welcome our new Editor Rohit Malhotra from Northwestern Medical Center, IL, USA, and we look forward to our cooperation and his input in the field of ACS and interventional cardiology. We would like to thank for substantial contributions Editor from Germany, well-known scientist Baktybek Kojonazarov and always will welcome him back.
Gulmira Kudaiberdieva
Editor-in-Chief
Heart, Vessels and Transplantation
Peer-review: Internal
Conflict of interest: None to declare
Authorship: G.K.
Acknowledgements and funding: None to declare
Statement on A.I.-assisted technologies use: We declare that we did not use AI-assisted technologies in preparation of this manuscript
Data and material availability: Does not apply
References
| 1.Scopus sources. Available at: URL: https://scopus.com/sources | ||||
| 2. Jiang C, Zhao Z, Yang Z, Wang Y, Xu Y, Xu H, et al. Dapagliflozin to reduce early recurrence after catheter ablation for atrial fibrillation: The DARE-AF randomize clinical trial. Circulation 2025; doi: 10.1161/CIRCULATIOINAHA.125.077447 | ||||
| 3. Sapp JL, Tang ASL, Parkash R, Stevenson WG, Healey J, Gula LJ, et al. for the VANISH 2 study. Catheter ablation or antiarrhythmic drugs for ventricular tachycardia. N Engl J Med 2025; 392: 737-47. https://doi.org/10.1056/NEJMoa2409501 PMid:39555820 |
||||
| 4. Wong CX, Cheung CC, Montenegro G, Oo HH, Pena IJ, Tang JJ, et al. Caffeinated coffee consumption or abstinence to reduce atrial fibrillation. The DECAF randomized controlled trial. JAMA 2025; doi: 10.1001. jama.2025.21056 https://doi.org/10.1001/jama.2025.21056 |
||||
| 5.Fearon WF, Terada K, Takahasi K, Skoda A, Luikart HI, Lamendola CA, et al. Cardiac allograft vasculopathy inhibition with alirocumab: the CAVIAR trail. Circulation 2025; doi: 10.1161/CIRCULATIOINAHA.125.077603 https://doi.org/10.1161/CIRCULATIONAHA.125.077603 PMid:41212178 |
||||
| 6. Beshish AG, Kwiatkowski DM, Sznycer-Taub N, Costello JM, Jergel A, et al. Hyperoxia during neonatal cardiopulmonary bypass is associated with worse clinical outcomes: A multi-institutional study. Circulation 2025; doi: 10.1161/CIRCULATIOINAHA.125.045890 https://doi.org/10.1161/circ.152.suppl_3.4358117 |
||||
| 8.Bohula EA, MArston NA, Bhatia AK, De Ferrari GM, Leiter LA, Nicolau JC, et al. Evolocumab, in patients without a previous myocardial infarction or stroke. N Engl J Med 2025; doi: 10.1056/nejmoa2514428 https://doi.org/10.1056/NEJMoa2514428 PMid:41211925 |
||||
| 9. Bi Y, Li M, Liu Y, Li T, Lu J, Duan P, et al. Intensive blood pressure control in patients with type 2 diabetes. N Engl J Med 2025; 392: 1155-67. https://doi.org/10.1056/NEJMoa2412006 PMid:39555827 |
||||
| 10. Van t Hof AW. CELEBRATE. Zalunfiban vs placebo at first medical contact in patients with STEMI? AHA sessions 2025. | ||||
| 11. Johansen ND, Modin D, Pardo-Secc J, Rodriguez-Tereiro-Sanchez, Loiacono MW, Harris RC, et al. High-dose vs standard-dose influenza vaccine and cardiovascular outcomes for older adults. Circulation 2025; doi: 10.1161/CIRCULATIOINAHA.125.077801 | ||||
| 12.Mach F, Koskinas KC, Roeters van Lennep JE, Tokgozoglu L, Badimon L, Baigent C, et al. 2025 focused update of 2019 ESC/EAS guidelines for the management of dyslipidemias: Developed by task force for the management of dyslipidemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2025; 46: 4359-78. https://doi.org/10.1093/eurheartj/ehaf190 PMid:40878289 |
||||
| 13.Galetti F, Farrar J, De Regil L. World Health Organization guidelines on the use and indications of glucagone like peptide-1 therapies for the treatment of obesity in adults. JAMA 2025; doi: 10.1001/jama.2025.24288 https://doi.org/10.1001/jama.2025.24288 |
||||
| 14. WHO. WHO updates list of essential medicines to include key cancer and diabetes treatments. 5 September 2025. Geneva. Available at URL: www.who.int/news/item/05-09-2025- who- updates- list- of- essential- medicines- to -include -key- cancer- | ||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

