Predictors of left ventricular hypertrophy development in patients with essential hypertension: role of pro- and anti-inflammatory cytokines
ORIGINAL RESEARCH ARTICLE
Predictors of left ventricular hypertrophy development in patients with essential hypertension: role of pro- and anti-inflammatory cytokines
Article Summary
- DOI: 10.24969/hvt.2018.86
- Page(s): 97-105
- CARDIOVASCULAR DISEASES
- Published: 24/10/2018
- Received: 07/03/2018
- Revised: 23/10/2018
- Accepted: 24/10/2018
- Views: 10609
- Downloads: 8671
-
Citations
- Keywords: cytokines, tumor necrosis factor-α, interleukin-10, essential hypertension, remodeling of the left ventricle
PDF PRINT VERSION Аннотация (Рус.) Корутунду (Кырг.) CommentsAddress for CorrespondenceAddress for correspondence: Larisa Tsoi, Kyrgyz-Russian Slavic University named after Boris Yeltsin, Bishkek, Kyrgyz
Republic; Email: lyularisa@mail.ru
Larisa G. Tsoi1, Andrei G. Polupanov1,2, Ibragim S. Sabirov1, Tazagul B. Zalova1, Flora T. Rysmatova31Kyrgyz-Russian Slavic University named after Boris Yeltsin, Bishkek, Kyrgyz Republic
2National Center for Cardiology and Therapy named after Academician M. Mirrakhimov at the Ministry of Health of the Kyrgyz Republic, Bishkek, Kyrgyz Republic
3Osh State University, Osh, Kyrgyz Republic
Abstract
Objective: The data on the relationship between the level of TNF-α and interleukin-10 with the presence of left ventricular hypertrophy and myocardial remodeling in patients with essential hypertension is presented.
Methods: Overall, 156 patients with essential hypertension aged 40 to 75 years (with mean age 55.8 ± 7.5 years) were examined; of which 57 were women and 99 were men. All patients were divided into two groups. The first group included 73 patients showing the presence of left ventricular hypertrophy (LVH), established by echocardiography; the second group included 83 patients who showed no signs of LVH on the echocardiogram.
Results: According to the study, no relationship was found between the concentration of tumor necrosis factor α (TNF-α) and the development of LVH. It was also shown that only men, but not women with, were associated with the presence of LVH with low levels of interleukin-10 ( IL-10). In addition, a negative correlation was found between the concentration of tumor necrosis factor α (TNF-α) and IL-10 with the thickness of the left ventricular walls at the initial stages of myocardial remodeling on the echocardiography.
Conclusion: Thus, our study demonstrates the modulating role of inflammation on the processes of myocardial remodeling in hypertension.
Key words: cytokines, tumor necrosis factor-α, interleukin-10, essential hypertension, remodeling of the left ventricle.
Introduction
Around the world, essential hypertension (EH) is among the leading places within the structure of cardiac pathology and, due to significant prevalence and early development of complications, is a complex medical and social problem. It is one of the main risk factors for cardiovascular diseases (CVD), primarily coronary heart disease (CHD) and mortality from CVD, whose share in the structure of total mortality exceeds 50% (1). With the so-called natural course of the disease, in 70% of the cases, it can end with cardiac complications (2), which underline the relevance of the research aimed at studying the state of the heart muscle in EH.
Currently, the term “hypertensive heart” is used to describe the complexity of structural and functional changes occurring in the myocardium during the EH (3). Left ventricular hypertrophy (LVH), being the main component of the hypertensive heart, significantly worsens the prognosis of the disease and is recognized as an independent risk factor for the development of heart failure, acute forms of CHD, arrhythmias and sudden death (4).
The main role in the occurrence of LVH is played by the hemodynamic factor. Numerous proofs exist, of a link between the frequency of LVH development and a degree of blood pressure increase (5.6). Moreover, the values of mean and systolic blood pressure measured during daily blood pressure monitoring, the degree of increase in morning blood pressure and the severity of its decrease at night (7) are important, unlike values of a “random” blood pressure measurement.
Hemodynamic parameters are not the only causes of LVH development. A certain role is assigned to genetic predisposition (8). An important role in the genesis of LVH is played by the neurohumoral influences (9), in particular; the sympathetic and renin-angiotensin systems, as well as aldosterone. According to Laragh (5), catecholamines and angiotensin II can serve as one of the main molecular mechanisms determining the development of myocardial hypertrophy in EH. Ganguly et al. (10) consider catecholamines to be the "hormones of myocardial hypertrophy". The experimental studies have shown that aldosterone contributes to the development of myocardial fibrosis, stimulation of the collagen synthesis, and indirectly influences the development of LVH, simulating local sympathetic activity (11).
Recent studies have shown a possible role of immuno-inflammatory activation mediated by pro-inflammatory cytokines in the development of organ lesions, including LVH, in EH. Cytokines are peptides that mediate cell-to-cell interactions through specific receptors on the cell surface. They camouflage themselves as immunocompetent cells such as, T-lymphocytes, macrophages, monocytes, and as non-immunocompetent cells (cardiomyocytes, endothelial cells). Cytokines manage the activation, differentiation, growth, death and effector functions of various cell types (12), which make them important factors in the pathophysiology of EH, the myocardial damage through this pathology, and the development of chronic heart failure. Despite the large number of experimental studies on the relationship of some cytokines (interleukin-6, tumor necrosis factor α -TNF-α) with the development of LVH, clinical studies on the effect of both pro and anti-inflammatory cytokines on the risk of LVH are not enough, and their results are rather ambiguous (13, 14).
Therefore, the purpose of our paper is to study the relationship between the level of TNF-α and interleukin-10 with the presence of LVH and myocardial remodeling in patients with essential hypertension.
Methods
Overall, 156 patients, aged 40 to 75 years (mean age 55.8±7.5 years) with essential hypertension (EH), were examined; of which 57 were women and 99 were men. The exclusion criteria for the study were patients with secondary forms of arterial hypertension (AH) who had myocardial infarction or cerebral stroke or an episode of unstable angina less than 6 months prior to inclusion to the study, patients with high functional class of chronic heart failure (NYHA III-IV), patients having hepatic or renal failure, cancer, acute inflammatory diseases or exacerbation of chronic inflammatory diseases within the last 2 weeks before inclusion to the study.
The patients were divided into 2 groups; first group included 73 patients with the presence of LVH, established through echocardiogram, and the second group included 83 patients who had no signs of LVH on the echocardiogram.
Clinical and biochemical measurements
The following examinations were performed on all patients: height, weight and waist volume measurements, blood pressure and heart rate measurements, as well as a number of biochemical parameters were determined: blood glucose and creatinine levels and lipid spectrum (total cholesterol- TC, low density lipoprotein cholesterol -LDL, high density lipoprotein cholesterol -HDL and triglyceride-TG levels).
Blood pressure (BP) was measured on both arms using the Korotkoff method with an aneroid sphygmomanometer in a sitting position, adhering to generally accepted pressure measurement rules (WHO, 1986). To assess overweight, height was measured using a stadiometer and weighed. The Quetelet index was calculated: weight (kg) / height (m)2. To identify individuals with abdominal obesity, waist circumference was measured at the midpoint of the distance between the costal arch and the iliac crest. With a waist circumference of more than 94 cm in men and more than 80 cm in women, abdominal obesity was diagnosed. Blood for the study was taken from the cubital vein in the sitting position in the morning on an empty stomach after a 12-hour night fasting.
The levels of glucose, TC, TG and HDL were determined on a Sinhron CX4-DELTA biochemical autoanalyzer (Beckman, USA). The concentration of LDL was calculated by Friedewald WT (1972)’s formula: LDL=TC-(TG/2.2)-HDL.
Echocardiography and Doppler Echocardiography
Echocardiographic (echo) test was carried out using Sequoia-512 apparatus of “Acuson” (USA) in a standard supine position of a patient. The test was performed within morning hours, on empty stomach after preliminary 15-minute rest. For evaluation of heart echo structures M- and B-scanning was carried out. Left ventricular (LV) end-diastolic (EDD) and end-systolic (ESD) chamber dimensions, left ventricular end-systolic (ESV) and end-diastolic (EDV) volume, posterior wall thickness of left ventricular (LV PWT) and ventricular septum (IVST), left ventricular ejection fraction (LVEF), left ventricular stroke volume (SV) were defined according to the standard methods.
Left ventricular myocardial mass (LVMM) was calculated according to the formula, proposed by Devereux and Reichek (1977):
LVMM = 1.04 x (EDD + IVST + LV PWT)3 – (EDD)3) – 13.6.
Left ventricular myocardium mass index (LVMMI) was defined by division of LVMM by the body surface area:
LVMMI=LVMM/S, where: S – body surface area (m2).
LVH was recognized in the case, if the posterior wall thickness of the left ventricular and/or the ventricular septum thickness were 12 mm and more, and the value of the LVMMI was equal or exceeded 115 g/m2 in men and 95 g/m2 in women.
A status of diastolic function was defined by transmitral flow curves by means of the pulse wave Doppler (Doppler echocardiographic device Sequoia - 512, “Acuson”, USA). The following indicators were estimated at evaluation of the left ventricular diastolic function: maximal early diastolic filling velocity (E’Vmax) – Е peak; maximal late diastolic filling velocity – А peak; ratio of transmitral blood flow velocities (Е/А); left atrial anterior- posterior dimension (L-P); LV isovolumic relaxation time (IVRT).
Determination of TNF-α and IL-10
TNF-α and IL-10 mass was determined by enzyme-linked immunosorbent assay by means of specialized test systems of “CYTOKINE-STIMUL-BEST”, Novosibirsk (Russia).
Statistical processing of study results
Statistical processing of obtained data was carried out by means of STATISTICA 6.0 program and by standard statistical programs. The normality of distribution was determined by Shapiro-Wilk and Lilliefors tests. The significance of differences between groups was defined by means of nonparametric Mann-Whitney and Kolmogorov-Smirnov tests and by means of parametric Student’s t-test. Interaction between indicators was studied by correlation analysis with calculation of Spearman’s correlation coefficient (r). For assessment of the predictive value of different factors in LV walls thickening, a multifactor regression analysis was used with step by step inclusion into the model. Differences were deemed to be significant at p<0,05.
Results
Characteristics of patients with/without left ventricular hypertrophy, included in the study
The data, presented in Table 1, shows that groups of patients were comparable by age, however, male patients (75% vs. 53% in the 1st group, p<0,01) prevailed among patients with LVH. Patients with LVH had higher body mass indices (32.4±5.1 kg/m2 vs. 29.8±4.0 kg/m2 in the 1st group, p<0.001), higher waist circumference (107±11.4 cm vs. 100±8.8 p<0.001), low level of of HDL cholesterol (0.78±0.14 mmol/L vs. 0.92±0.24 mmol/L, p<0,001), higher glomerular filtration rate (104±41 ml/min vs. 91±29 ml/min p<0.01), and suffered from obesity (75.3% vs. 42.1%, p<0.01) as compared to patients without LVH.
At the same time, according to the level of systolic (SBP) and diastolic (DBP) blood pressure, blood glucose level, indicators of lipid profile, fibrinogen and creatinine level, the groups did not differ significantly (p>0. 05) (Table 1). It is to be noted that there were no differences between groups of patients without/with LVH in the incidence of CHD (45.7% and 41.1%, respectively, p>0.05) and carotid atherosclerosis (73.4% and 64.4%, respectively, p>0.05) (Table 1).
Table 1. Clinical characteristics of patients, included in the study
Parameters
LVH – (Group 1)
LVH+ (Group 2)
р
Age, years
55.5±7.5
56.2±7.8
NS
Gender m/f, n(%)
44/39 (53)
55/18 (75)
<0.01
SBP, mm Hg
160±28
166±23
NS
DBP, mm Hg
96±13
99±11
NS
BMI, kg/m2
29.8±4.0
32.4±5.1
<0.001
Obesity
35 (42.1)
55 (75.3)
<0.01
WC, cm
100±8.8
107±11.4
<0.001
AO, n(%)
56 (67.5)
57 (78.1)
NS
Glucose, mmol/L
5.25±1.09
5.11±1.44
NS
TC, mmol/L
5.30±1.15
5.02±1.10
NS
LDL cholesterol, mmol/L
3.39±0.87
3.25±1.11
NS
HDL cholesterol, mmol/L
0.92±0.24
0.78±0.14
<0.001
TG, mmol/L
2.12±1.08
1.98±0.76
NS
Fibrinogen, mg/L
4244±1111
4315±1418
NS
Creatinine, µmol/L
90±23
107±84
NS
CC, ml/min
91±29
104±41
<0.01
CHD, n(%)
38 (45.7)
30 (41.1)
NS
CA, n(%)
61 (73.4)
47 (64.4)
NS
Data are presented as mean ± SD, number and percentage
AO – abdominal obesity, BMI – body mass index; CA – carotid atherosclerosis, CC – creatinine clearance, CHD – coronary heart disease, DBP - diastolic blood pressure, HDL – high-density lipoprotein cholesterol, LDL – low-density lipoprotein cholesterol, LVH – left ventricular hypertrophy, NS – not significant, SBP- systolic blood pressure, TC – total cholesterol, TG – triglycerides; WC – waist circumferenceEchocardiographic and Doppler echocardiographic indicators in patients with/without left ventricular hypertrophy
Echo and Doppler echocardiography results for patients of both groups are presented in Table 2. As expected, significantly higher values of LV wall thickness, LV myocardium mass, as well as increase of left heart cavities and its volume at systole and diastole were registered in patients with LVH in comparison with patients with EH without LVH (p<0.001) (Table 2). Some reduction of LVEF in patients with EH with LVH (60.3±6.9% vs. 62.9±5.1%, p<0.01) as compared to patients without LVH, was observed.
The analysis of the LV diastolic function in both groups showed that patients with LVH had more evident signs of diastolic dysfunction of myocardium in comparison with those without LVH. Thus, significantly larger size of left atrium (LA) (3.68±0.42 cm vs. 3.37±0.44 cm in patients of the 1st group, p<0.01), and increase of IVRT (84±26 ms vs. 75±18 ms, p<0.05) was characteristic for patients with LVH as compared to those without LVH (Table 2). As for the value of relative thickness of LV myocardium walls and the level of pulmonary arterial pressure (PAP), groups were comparable (p>0.05).
TNF-α and Interleikin-10 Concentration in Patients with Essential Hypertension with/without Left Ventricular Hypertrophy
The data presented in the Figure 1 below shows that, TNF-α concentration in patients without LVH, equal to 8.43±1.36 pg/ml, appeared to be comparable with concentration of such cytokine in patients with EH with LVH (8.54±1.58 pg/ml, p>0.05). The indicated trend was illustrative both for males and females (Fig. 1).
IL-10 concentration appeared also to be comparable in both groups (15.4±3.6 pg/ml in the 1st group and 14.7±3.4 pg/ml in the 2nd group, p>0.05). However, we have revealed sex peculiarities in correlation of IL-10 level in patients with LVH with EH. Thus, if cytokine concentration in females in groups with/without LVH did not significantly differ, IL-10 level in males without LVH, equal to 15.7±3.6 pg/ml, appeared to be significantly higher than the value of similar indicator in the group of males with LVH (14.8±2.9 pg/ml, p<0.025) (Fig. 1).
Table 2. Echocardiographic and Doppler echocardiography data of patients with essential hypertension with/without left ventricular hypertrophy
Parameters
LVH – (Group 1)
LVH+ (Group 2)
р
Number of patients, n
83
73
-
IVST, cm
0.95±0.08
1.12±0.16
<0.001
LV PWT, cm
0.96±0.08
1.12±0.14
<0.001
RWT, units
0.39±0.05
0.41±0.08
NS
EDD, cm
4.93±0.49
5.54±0.48
<0.001
ESD, cm
3.35±0.74
3.69±0.45
<0.001
EDV, ml
116±24
152±29
<0.001
ESV, ml
44±14
58±16
<0.001
LVMM, g
179±35
284±65
<0.001
SV, ml
73±16
90±20
<0.001
EF, %
62.9±5.1
60.3±6.9
<0.01
LA, cm
3.37±0.44
3.68±0.42
<0.001
Е, cm/s
0.67±0.17
0.63±0.16
NS
А, cm/s
0.71±0.17
0.66±0.13
NS
Е/А, units
0.94±0.30
0.88±0.30
NS
IVRT, ms
75±18
84±26
<0.05
PAP, mmHg
20.5±3.9
20.5±4,0
NS
Data are presented as mean ± SD
А - maximal late diastolic filling velocity, Е -maximal early diastolic filling velocity; Е/А - ratio of transmitral blood flow velocities, EDD – left ventricular end-diastolic dimension, EDV - left ventricular end-diastolic volume, ESD - left ventricular end-systolic dimension, ESV - left ventricular end-systolic volume, IVRT – left ventricular isovolumic relaxation time, IVST – interventricular septal thickness, LA - left atrial anterior- posterior dimension, LVEF - left ventricular ejection fraction, LVMM – left ventricular myocardial mass, NS - not significant, LV PWT –left ventricular posterior wall thickness, PAP – pulmonary artery pressure, RWT – relative wall thickness, SV - left ventricular stroke volume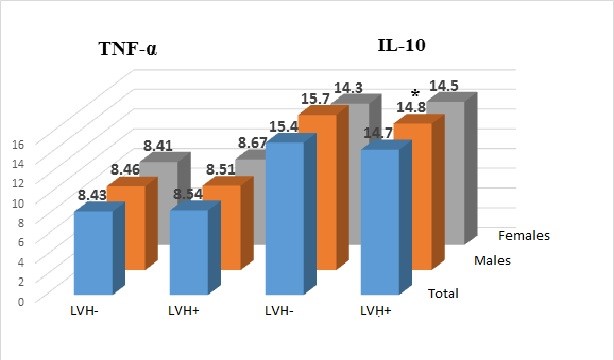
Figure 1 . TNF-α and Interleikin-10 concentration in patients with essential hypertension with/without left ventricular hypertrophy, * - p<0.025, IL-10 – interleikin 10, LVH – left ventricular hypertrophy, TNF –a – tumor necrosis factor α
Correlation of blood serum cytokines concentration with structural functional condition of left heart in patients with essential hypertension with/without left ventricular hypertrophy
Evaluating the association of cytokines concentration with structural-functional condition of the left heart, we have obtained the following results. It appeared that TNF-α concentration in the total group correlates significantly negatively with IVST (r=-0,29; p<0.001), LV PWT (r=-0,29; p<0.001) and RWT (r=-0,30; p<0.001). Besides, correlation was identified between the cytokines levels with LV diastolic function. In particular, there was a positive correlation with the E peak (r=0.21; p<0.05), E/А ratio (r=0.21; p<0.05) and a negative association with IVRT (r=-0.21; p<0.05).
As for IL-10, negative correlation with RWT was observed (r=-0.23; p<0.01) and positive association with the LV EDD size (r=0.29; p<0.001), and also with the SV (r=0.17; p<0.05).
Thus, we were interested to investigate the correlation of the cytokines concentration with the condition of the left heart separately in groups of patients with/without LVH. The obtained results are indicated in Table 3.
Table 3. Factors, associated with myocardial walls thickening in patients with essential hypertension without evident hypertrophy of left ventricular myocardium
Variables
r
p
β
p
IVST
Age
0.09
NS
-
-
BMI
0.18
NS
0.29
<0.025
WC
0.36
<0.001
0.45
<0.001
SBP
0.23
<0.05
-
-
DBP
0.36
<0.001
0.29
<0.01
CC
0.06
NS
-
-
TNF-α
-0.50
<0.0001
-0.48
<0.0001
IL-10
-0.20
=0.06
-0.34
<0.001
LV PWT
Age
0.25
<0.025
0.24
<0.01
BMI
0.12
NS
-
-
WC
0.27
<0.01
0.29
<0.01
SBP
0.27
<0.01
-
-
DBP
0.32
<0<005
0.19
<0.05
CC
-0.14
NS
-
-
TNF-α
-0.46
<0.001
-0.38
<0.001
IL-10
-0.33
<0<001
-0.36
<0.001
BMI – body mass index, β – multiple regression coefficient, CC – creatinine clearance, DBP – diastolic blood pressure, IL-10 – interleukin-10, IVST – interventricular septum thickness, LVPWT – left ventricular posterior wall thickness, NS – not significant, r – correlation coefficient, SBP – systolic blood pressure, TNF-α – tumor necrosis factor α, WC – waist circumference
It can be observed from the table 3 that LV wall thickness in patients without evident myocardial hypertrophy had a significantly negative correlation both with TNF-α concentration (for IVST: r=-0.50; p<0.0001; for LV PWT: r=-0.46; p<0.001), and with IL-10 level (for IVST: r=-0.20; p=0.06; for LV PWT: r=-0.33; p<0.001). Besides, significant correlation was noted between the myocardial walls thickness with the waist circumference and levels of systolic and diastolic blood pressure (Table 3).
The adjustment by age, SBP and DBP level, body mass index, waist circumference and glomerular filtration rate with conduction of the multifactor regression analysis confirmed the independence of association of TNF-α and IL-10 levels with LV walls thickness in patients with EH at initial stages of myocardial remodeling. Both TNF-α and IL-10 acted as antirisk factors of myocardial walls thickening at early stages of LVH development.
Thus, for TNF-α a multiple regression coefficient was (-0.48) for IVST (p<0.001) and -0.38 for LV PWT (p<0.001). Similarly for IL-10, a significant inverse correlation was identified with IVST (β=-0.34; p<0.001) and LV PWT (β=-0.36; p<0.001). On the contrary, independent risk factors for LV walls thickening were; age, BMI, waist circumference and a level of DBP (Table 3).
A different situation was observed in patients with EH with the presence of clear LVH. In this group of patients, a significant association of the level of TNF-α with the thickness of the walls of the myocardium (r = 0.13; p> 0.05) was not revealed. At the same time, as in the patients in the 1st group, we found a negative correlation of the concentration of IL-10 with the IVST (r = -0.23; p <0.05). However, after adjusting for other risk factors for LVH (age, level of blood pressure and BMI), this association was not significant (β = -0.17; p> 0.05).
Discussion
Given the important role of subclinical inflammation in the development and progression of heart failure, the main goal of our study was to study the relationship of TNF-α and IL-10 with the presence of left ventricular hypertrophy and myocardial remodeling in patients with essential hypertension. However, no relationship was found between the concentration of TNF-α, and the development of LVH. It was also shown that only for men did the low levels of IL-10 are associated with the presence of LVH but not for women. In addition, a negative correlation was found between the concentration of TNF-α and IL-10 with the thickness of the LV walls at the initial stages of myocardial remodeling in the EH. Thus, our study demonstrates the simulating role of inflammation on the processes of myocardial remodeling in hypertension.
Recent studies have shown the important role of cytokines in the pathophysiology of EH and its complications, in particular in the development of heart failure. Experimental studies have shown that cytokines contribute to myocardial remodeling, stimulating the synthesis of sarcomere proteins, disrupting intracellular calcium homeostasis, changing the degradation of the extracellular matrix and initiating apoptosis (13,14). Although most of the circulating cytokines are camouflaged by activated macrophages and lymphocytes, cardiomyocytes, adipocytes, and endothelial cells can be sources of these biomolecules (15). Pro-inflammatory cytokines are not continuously synthesized in the myocardium, and are expressed in response to damage, which leads to their increase in circulation (16).
Among pro-inflammatory cytokines, in the context of EH, TNF-α deserves much attention. Firstly, as shown by experimental studies, hemodynamic stress caused by an increase in blood pressure is one of the incentives for increasing the production of pro-inflammatory cytokines, including TNF-α (17). Secondly, the ability of the cytokine to simulate the structure and function of the cardiovascular system was demonstrated, in particular, to reduce myocardial contractility, change the intracellular calcium homeostasis, and initiate cardiomyocyte hypertrophy and interstitial fibrosis (15). In addition, TNF-α promotes apoptosis of cardiomyocytes, and also activates metalloproteinases and disrupts the expression of their inhibitors, possibly contributing to cardiac remodeling (18).
A number of studies have demonstrated the hypertrophic effects of TNF-α in patients with EH (13,19), metabolic syndrome (20), chronic kidney disease (21), and also in uremic and dialysis patients (22). At the same time, it should be noted that the literature data on this issue is ambiguous. In particular, Jastrzebski M et al. (2006) did not reveal the relationship of the level of TNF-α in the presence of echocardiographically defined LVH (23). Similar data were obtained by Malavazos et al. for patients with metabolic syndrome (24), and Masiha et al. for elderly hypertensive patients (25). Leibowitz et al. believe that "... hypertensive patients with LVH do not always demonstrate elevated levels of cytokines" (26).
In our study, there was also no association of plasma levels of TNF-α with LVH in patients with EH. However, it should be emphasized that the lack of an increase in TNF-α does not exclude the presence of an active level of this cytokine in the plasma, since its soluble receptors, which change during EH (27), may hinder or even inhibit the determination of TNF-α in plasma using the enzyme immunoassay (28). In addition, when considering the functional activity of TNF-α, the definition and study of the role of its soluble receptors (rTNF-P), which are considered as indicators reflecting the synthesis, and possibly the biological activity of TNF-α, is of undoubted interest (29). It depends on relative concentration and clearance rate of its components, and first of all, on the disbalance between the synthesis of TNF-α and rTNF-P (30).
It is supposed that TNF-α, a membrane and a soluble form of TNF-P, constitute a uniform biological system, in which the TNF-α has functional activity. Considering the issue from this point of view helps to understand the negative correlation between the level of TNF-α and myocardial wall thickness that we identified in EH patients in the early stages of myocardial remodeling prior to the development of clear LVH. It is possible to assume that the enhancement of cytokine production as myocardial stress increases in the initial stages of cardiac remodeling is accompanied by an increased expression of its soluble receptors. In this case, increasing the synthesis of rTNF-P can be regarded as an adaptive mechanism, which, on the one hand, reduces the number of active receptors on the cell surface, and on the other hand, by binding to TNF-α, soluble forms of receptors can neutralize its biological activity. Therefore, we believe that in our case there is no true decrease in the activity of TNF-α in the initial stages of LVH, namely, an increase in the expression in the myocardium of its soluble receptors, which neutralize the activity of the cytokine. An indirect confirmation of our assumption we find in the study Kovaleva et al. (27), who showed that an increase in the plasma concentration of rTNF-P is observed in the early stages of the EH.
IL-10 is one of the main anti-inflammatory cytokines and one of the most sensitive markers of subclinical inflammation in cardiovascular diseases. Reducing the secretion of pro-inflammatory cytokines (TNF-α IL-6, IL-1), IL-10 thereby limits the excessive immune response (31). Studies on the role of IL-10 in myocardial remodeling in the EH are scarce. In particular, a high concentration of IL-10 was established with myocardial remodeling after acute myocardial infarction, including hypertensive individuals (32). We have demonstrated a decrease in the level of IL-10 in men with pronounced LVH. Similar results are reported by Yilmaz et al., who, in his study on nondiabetic patients on hemodialysis, found a negative association between the severity of LVH and the level of IL-10 (22). Apparently, against the background of pronounced myocardial damage and the development of its hypertrophy, a shift in the balance between pro- and anti-inflammatory cytokines takes place towards the predominance of the first with an increase in inflammatory and apoptotic changes in the myocardium, which is one of the mechanisms of progression of cardiac dysfunction and the development of heart.
Study limitations
Our study has some limitations, in particular, because it is a cross-sectional observational study and does not allow to assess the influence of cytokines on the dynamics of echocardiographic indicators. Secondly, unavailability of data on TNF-α soluble receptors does not allow to evaluate their influence on TNF-α activity and on the effectiveness of the activity and the nature of responses of this whole biological system.
Conclusion
Thus, our study demonstrates the modulating role of inflammation on the processes of myocardial remodeling in hypertension.
Peer-review: external and internal
Conflict of interest: None to declare
Authorship: T.L.G., P.A.G., S.I.S, Z.T.B., R.F.T equally contributed to the study and preparation of manuscript
Acknowledgement and funding: None to declare
References
1. Lazzini A., Lazzini S. Cardiovascular disease: an economical perspective. Curr Pharm Des 2009; 15: 1142-56. https://doi.org/10.2174/138161209787846883 2. Shhvatsabaya I.K., Yurenev A.P. Hypertensive heart. Kardiologia. 1988; 5: 72-6. 3. Devereux RB, de Simone G, Ganau A, Koren MJ, Mensah GA, Roman MJ. Left ventricular hypertrophy and hypertension. Clin Exp Hypertens 1993; 15: 1025-32. Review. https://doi.org/10.3109/10641969309037090 PMid:8268870 4. Levy D, Anderson KM, Savage DD, Balkus SA, Kannel WB, Castelli WP. Risk of ventricular arrhythmias in left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol 1987; 60: 560-5. https://doi.org/10.1016/0002-9149(87)90305-5 5. Laragh G. Cardiac pathophysiology and its heterogeneity in patients with established hypertensive disease. Ibid 1988; 84: 3-11. 6. Shliahto EV, Konradi AO, Zaharov DV, Rudomanov OU. Structural and functional changes in myocardium in patients with hypertension. Kardiologia 1999; 2: 49-55. 7. Gosse P, Campello G, Aouizerate E, Roudaut R, Dallocchio M. [Left ventricular hypertrophy of the hypertensive patient: relation to exertional and ambulatory blood pressure]. Arch Mal Coeur Vaiss 1986;79: 796-800. PMid:2948469 8. Koren MJ, Mensah GA, Blake J, Laragh JH, Devereux RB. et al. Comparison of left ventricular mass and geometry in black and white patients with essential hypertension. Am J Hypertens 1993; 6: 815-23.https://doi.org/10.1093/ajh/6.10.815 PMid:8267936 9. Parfenova E.V., Diakonava E.G., Miasianenko V.I. i drugie. Blood levels of hormones, neuromediators and left ventricular hypertrophy in patients with hypertension. Kardiologia 1995; 7: 18-24. 10. Ganguly PK, Lee SL, Beamish RE, Dhalla NS. Altered sympathetic system and adrenoceptors during the development of cardiac hypertrophy. Am Heart J 1989; 118: 520-5. https://doi.org/10.1016/0002-8703(89)90267-6 11. Weber KT, Brilla CG. Factors associated with reactive and reparative fibrosis of the myocardium. Basic Res Cardiol 1992; 87 Suppl 1: 291-301. PMid:1497573 12. Simbirtsev AS. Cytokines in pathogenesis of infectious and noninfectious diseases in human.. Meditsinskiy akademicheskiy jurnal. 2013; 3: 18-41. 13. Aksenova TA. [The immunologic disorders and dysfunction of endothelium as predictors of development of hypertrophy of left ventricle of heart in patients with hypertension disease]. Klin Lab Diagn 2013; 8: 18-20. 14. Cai JY, Zhai GL, Gao W, Zhu L, Li Y. [A study of the relationship between remodeling of left ventricle and endothelial injury and pro-inflammatory mediators in different stages of essential hypertension]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008; 20: 743-5. PMid:19111125 15. Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell 1999; 97: 189-98.https://doi.org/10.1016/S0092-8674(00)80729-1 16. Volkova SYu. Diagnostic capabilities of several neurohumoral systmes in definition of left ventricular systolic dysfunction in CAD patients with heart failure. Serdechnaya nedostatochnost 2008; 1: 25-30. 17. Goldhaber JI, Kim KH, Natterson PD, Lawrence T, Yang P, Weiss JN. Effects of TNF-alpha on [Ca2+]i and contractility in isolated adult rabbit ventricular myocytes. Am J Physiol 1996; 271(4 Pt 2): H1449-55. PMid:8897939 18. Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, et al. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci U S A 2000; 97: 12746-51. https://doi.org/10.1073/pnas.97.23.12746 PMid:11070088 PMCid:PMC18835 19. Cai JY, Zhai GL, Gao W, Zhu L, Li Y. [A study of the relationship between remodeling of left ventricle and endothelial injury and pro-inflammatory mediators in different stages of essential hypertension]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008; 20: 743-5. PMid:19111125 20. Sciarretta S, Ferrucci A, Ciavarella GM, De Paolis P, Venturelli V, Tocci G, et al. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens 2007; 20: 784-91. https://doi.org/10.1016/j.amjhyper.2007.01.023 PMid:17586414 21. Cottone S, Nardi E, Mulè G, Vadalà A, Lorito MC, Riccobene R, et al. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol 2007; 67: 209-16.https://doi.org/10.5414/CNP67209 PMid:17474556 22. Yilmaz R, Altun B, Ozer N, Hazirolan T, Turgan C. et al. Impact of cytokine genotype on cardiovascular surrogate markers in hemodialysis patients. Ren Fail. 2010; 32: 806-16. https://doi.org/10.3109/0886022X.2010.494798 PMid:20662694 23. Jastrzebski M, Czarnecka D, Rajzer M, Kawecka-Jaszcz K. Increased levels of inflammatory markers in hypertensives with target organ damage. Kardiol Pol 2006; 64: 802-9. PMid:16981055 24. Malavazos AE, Corsi MM, Ermetici F, Coman C, Sardanelli F, Rossi A, et al. Proinflammatory cytokines and cardiac abnormalities in uncomplicated obesity: relationship with abdominal fat deposition. Nutr Metab Cardiovasc Dis 2007; 17: 294-302. https://doi.org/10.1016/j.numecd.2006.01.001 PMid:17434052 25. Masiha S, Sundström J, Lind L. Inflammatory markers are associated with left ventricular hypertrophy and diastolic dysfunction in a population-based sample of elderly men and women. J Hum Hypertens 2013; 27: 13-7.https://doi.org/10.1038/jhh.2011.113 PMid:22237633 26. Leibowitz D, Planer D, Ben-Ivgi F , Weiss AT, Bursztyn M. Tumor necrosis factor and interleukin-6 levels in hypertensive patients with and without left ventricular hypertrophy. Blood Press 2005; 14: 21-4.https://doi.org/10.1080/08037050410004792 PMid:15823943 27. Kovaleva O.N., Asheulova T.V., Gerasimchuk N.N. Relationship between immune activation and oxidative stress in patients with hypertension and their correction with combined antihypertensive therapy. Nauchnie vedomosti; 2015; 16: 52-59. 28. Kovaleva O.N., Ambrosova T.N. Cytokines: common biologic and cardiac effects. Harkov; 2006. PMCid:PMC1563863 29. Tilz GP, Diez-Ruiz A, Baier-Bitterlich G, Demel U, Wachter H, Fuchs D. Soluble receptors for tumor necrosis factor and neopterin as parameters of cell-mediated immune activation. Hematology 1996; 1: 141-54.https://doi.org/10.1080/10245332.1996.11746298 PMid:27406429 30. Weckmann AL, Alcocer-Varela J. Cytokine inhibitors in autoimmune disease. Semin Arthritis Rheum 1996; 26: 539-57. https://doi.org/10.1016/S0049-0172(96)80042-4 31. Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ Res 2009; 104: e9-18. https://doi.org/10.1161/CIRCRESAHA.108.188243 PMid:19096025 PMCid:PMC2774810 32. Zarrouk-Mahjoub S, Zaghdoudi M, Amira Z, Chebi H, Khabouchi N, Finsterer J, et al. Pro- and anti-inflammatory cytokines in post-infarction left ventricular remodeling. Int J Cardiol 2016; 221: 632-6.https://doi.org/10.1016/j.ijcard.2016.07.073 PMid:27423081 Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.Archive of Issues
AUTHOR'S CORNER
 Authors having problems with submissions please notify editor: editor@hvt-journal.com
Authors having problems with submissions please notify editor: editor@hvt-journal.com
