The prevalence and course of COVID-19 and the cardiovascular diseases
REVIEW
The prevalence and course of COVID-19 and the cardiovascular diseases
Article Summary
- DOI: 10.24969/hvt.2022.329
- Page(s): 124-135
- CARDIOVASCULAR DISEASES
- Published: 25/06/2022
- Received: 11/01/2021
- Revised: 14/06/2022
- Accepted: 16/06/2022
- Views: 6500
- Downloads: 4718
- Keywords: Covid 19; complications, cardiovascular diseases, prevalence
Address for Correspondence: Narendra Kumar, James Paget University Hospital, Lowestoft Road, Gorleston-on-Sea, Great Yarmouth NR31 6LA, UK Email: drnarendra007kr@gmail.com
1Jacky Kit Chan, 2Omar Assaf, 3Elhosseyn Guella, 4Shaima Mustafa, 5Narendra Kumar
1Pro Care Heart Clinic, Hong Kong
2Lancashire Cardiac Centre, Blackpool Victoria Hospital, Whinney Heys Rd, Blackpool FY3 8NR, UK.
3Dept of Cardiology, Manchester Royal infirmary, Oxford Road, Manchester M14 9WP
4Benha University, Faculty of Medicine, Egypt.
5James Paget University Hospital, Lowestoft Road, Gorleston-on-Sea, Great Yarmouth NR31 6LA, UK
Abstract
The SARS-CoV2 virus has infected over 545 million people and has claimed over 6 million lives globally by the end of June 2022.
The global case fatality rate ranged from 5.5% in Mexico, 2.1-2.5% in South Africa, Brazil, Guatemala, Russia, 1.1-1.5% in the United States, Canada, India and Chile, 0.5-0.9% in Western European countries, 0.3% in Japan, 0.2% in Taiwan to 0.1% in Australia.
Among hospitalized COVID-19 patients, the prevalence of pre-existing cardiovascular diseases was approximately 10%. The prevalence of cardiovascular diseases among COVID-19 non-survivors and COVID-19 patients requiring intensive care unit admission were approximately 20-30%.
In a multicenter study of 8910 COVID-19 patients from 169 hospitals in Asia, Europe and North America, presence of pre-existing coronary artery disease, chronic heart failure and cardiac arrhythmia was associated with increased mortality of 10.2%, 15.3% and 11.5% respectively, compared with 5-6% mortality in those without the above co-morbidities.
The systemic inflammation caused by SARS-CoV-2 could lead to a wide spectrum of cardiovascular complications including acute cardiac injury, acute coronary syndrome, coronary artery dissection, acute myocarditis, cardiomyopathy, chronic heart failure, cardiac arrhythmia, pulmonary embolism, cardiogenic shock, circulatory failure or even cardiac arrest.
Key words: Covid 19; complications, cardiovascular diseases, prevalence
Introduction
The World Health Organization has announced Coronavirus Disease 2019 (COVID-19) as a global pandemic in March 2020 (1).
The SARS-COV2 virus has infected over 545 million people and has affected over 6 million lives globally by the end of June 2022 (2).
According to the Johns Hopkins University Corona Virus Resource Center mortality analysis, the global case fatality rate (CFR) ranged from 5.5% in Mexico, 2.1-2.5% in South Africa, Brazil, Guatemala, Russia, 1.1-1.5% in the United States, Canada, India and Chile, 0.5-0.9% in Western European countries, 0.3% in Japan, 0.2% in Taiwan to 0.1% in Australia (2).
In mainland China, the CFR ranged from 5.8% in Wuhan to 0.7% in the rest of China (3). The adjusted CFR in mainland China was about 1.38% according to a model-based analysis (4). The CFR increased with age, ranging from < 1% in patients younger than 60 years old to 14.8-20% in patients older than 80 years old (3, 5, 6).
Prevalence of cardiovascular disease (CVD) in COVID-19 patients
In a multicenter study of 8910 COVID-19 patients, the prevalence of coronary artery disease (7) was 10.8% and 20% among survivors and non-survivors respectively. The prevalence of congestive heart failure (CHF) was 1.9% and 5.6% among survivors and non-survivors respectively. In a study of 72314 confirmed, suspected and asymptomatic COVID-19 patients in China, the prevalence of CVD was 4.2% (8).
In a meta-analysis of 1576 COVID-19 patients, the prevalence of CVD was 8.4% (9). The prevalence of CVD was even higher among hospitalized COVID-19 patients (8 -14.5%) (10, 11) and COVID-19 patients requiring intensive care unit (ICU) admission (25%) (12). According to the Center of Disease Control (CDC) and Prevention Morbidity and Mortality Weekly Report (MMWR) on 3rd April 2020, among 74439 hospitalized and non-hospitalized COVID-19 patients, the prevalence of underlying CVD was 9%. The prevalence of CVD among hospitalized COVID-19 patients and those requiring ICU admission were 23% and 29% respectively (12) .
Cardiovascular (CVD) and COVID-19 CFR
In a cohort of 72314 COVID-19 patients reported by the Chinese Center for Disease Control and Prevention, the overall CFR was 2.3% (1023 deaths among 44 672 confirmed cases). Presence of pre-existing CVD increased COVID-19 CFR to 10.5% (5). In a multicenter study of 8910 COVID-19 patients from 169 hospitals in Asia, Europe and North America, presence of pre-existing coronary artery disease (CAD), chronic heart failure (CHF) and cardiac arrhythmia was associated with increased mortality of 10.2%, 15.3% and 11.5% respectively, compared with 5-6% mortality in those without the above co-morbidities.
Pathophysiological linkage between COVID-19 infection and CVD
Angiotensin converting enzyme 2 (8) (ACE-2) converts angiotensin I and angiotensin II to angiotensin (1-9) (Ang 1-9) and angiotensin (1-7) (Ang 1-7) respectively. Angiotensin (1-7) acts on Mas-1 receptor and triggers nitric oxide release, reduces the release of pro-inflammatory tumor necrosis factor-α and interleukin-6 (Fig. 1). Therefore, ACE2 could mediate its cardioprotective effect by inhibiting cellular inflammation, fibrosis, cellular proliferation and vasoconstriction (13, 14). ACE-2 (8) (8) receptors are expressed in the lungs, heart, arterial / venous endothelium, gastrointestinal tract and kidneys (15, 16). They are upregulated in patients with CHF (17, 18), myocardial infarction (19) and valvular heart diseases (18). ACE-2 (8) (8) expression is enhanced in cardiomyocytes and endothelial cells in patients with heart diseases (19, 20).
COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SAR CoV-2), which uses its surface spike protein to bind ACE2 receptors for host cells entry and viral replication (21). Binding of SARS-CoV-2 to ACE2 receptors results in disruption and down-regulation of ACE2, which upregulates inflammatory cytokines, and promotes systemic inflammation (22). Enhanced myocardial and endothelial expression of ACE2 receptors in patients with CVD promotes SARS-CovV-2 cellular binding and entry, which in turn triggers the cascade of systemic inflammatory response and myocardial injury.
SARS-CoV-2 anchors on ACE 2 (8) receptors to enter the host cells including pneumocytes, macrophages, endothelial cells, pericytes and cardiac myocytes. Binding of SARS-CoV-2 to ACE2 receptors on the above host cells results in disruption and down-regulation of ACE2, which upregulates inflammatory cytokines (22) and triggers a cascade of systemic inflammation and multiorgan failure. Infection of endothelial cells and cardiac myocytes could lead to vascular inflammation, microvascular / macrovascular dysfunction, myocardial damage, and myocarditis. Immune over-reactivity and vascular inflammation could destabilize atherosclerotic plaques, triggering plaque rupture and acute coronary syndromes. Invasion of macrophages by SARS-CoV-2 could also lead to release of inflammatory cytokines including interleukin 6 (IL-6), interleukin 7 (IL-7), interleukin 22 (IL-22) and CXCL10. The cytokine storm and infiltration of T cells and macrophages into infected myocardium could lead to fulminant myocarditis and severe myocardial damage. Myocardial damage could in turn precipitate decompensated heart failure and cardiac arrhythmia.
Cardiovascular complications of COVID-19 infection
The systemic inflammation caused by SARS-CoV-2 could lead to a wide spectrum of cardiovascular complications including acute cardiac injury, acute coronary syndrome, coronary artery dissection, acute myocarditis, cardiomyopathy, CHF, cardiac arrhythmia, pulmonary embolism, cardiogenic shock, circulatory failure or even cardiac arrest (23, 24).
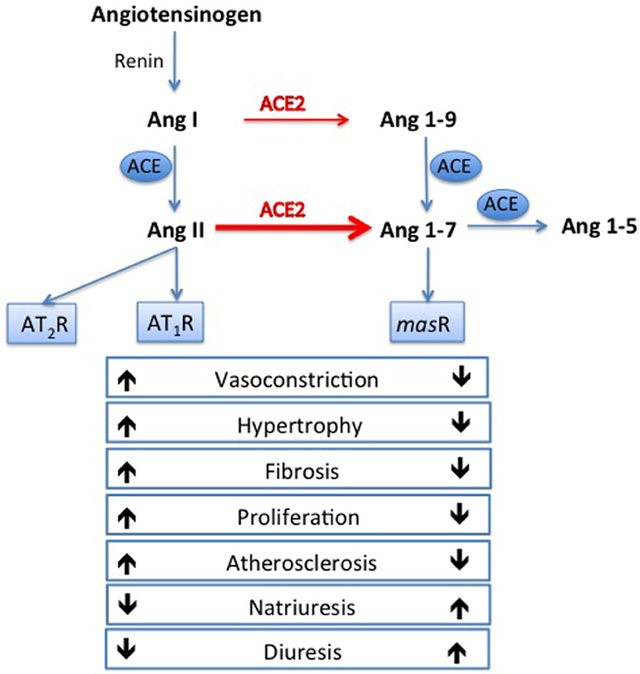
Figure 1. Renin-angiotensin system and actions of angiotensin converting enzyme (ACE) and ACE2. ACE converts angiotensin I to angiotensin II. Angiotensin II activates angiotensin II type 1 receptor (AT1) and triggers vasoconstrictive, proinflammatory, profibrotic, prothrombotic and arrhythmogenic responses. ACE 2 (8) converts angiotensin I and angiotensin II to angiotensin 1-9 and angiotensin 1-7 respectively. Thus, ACE2 exerts its cardioprotective actions by activating Angiotensin 1-7, Mas-1 receptor, triggering nitric oxide release and a cascade of vasodilatory, antiproliferative, anti-inflammatory, antifibrotic and antithrombotic responses (Modified from ref 19. Published under CC-BY licence).
Acute cardiac injury
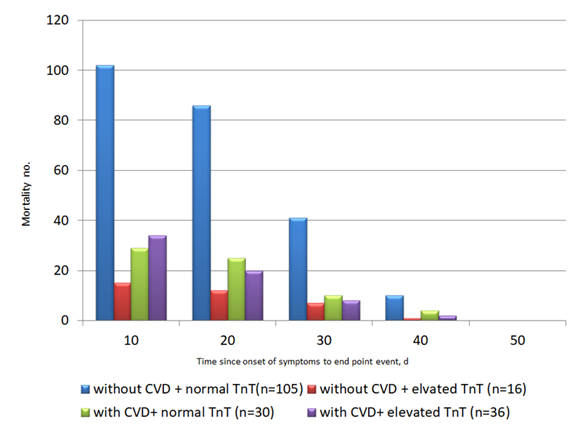
Acute cardiac injury (ACI) is defined as cardiac troponin elevation > 99% of the upper limit of normal, with or without abnormal electrocardiographic or echocardiographic changes. The prevalence of ACI was estimated to be between 7% to 20% among all hospitalized COVID-19 patients. The prevalence was as high as 22% among COVID-19 patients requiring ICU admission. Among COVID-19 non-survivors, the prevalence of ACI was almost 60 times higher than that in COVID-19 survivors (59% vs 1 %; p<0.0001) (11, 12, 21, 25). COVID-19 patients with underlying CVD were more likely to exhibit elevation of cardiac troponin T levels compared with those without CVD (54.5% vs 13.2%) (26). Patients with ACI after COVID-19 infection carry poor prognosis (Fig. 2). The study by Zhou et al (11) demonstrated that among 191 hospitalized COVID-19 patients, 17% developed ACI. The high-sensitive cardiac troponin I (hs-cTnI) levels raised from 8.8 pg/ml on admission to 290.6pg/ml on day 22 among the non-survivors, while the hs-cTnI levels remained static among the survivors. Guo et al. (26) reported a series of 187 hospitalized COVID-19 patients. The mortality of patients with cardiac troponin T (TnT) elevation and pre-existing CVD was 9 times higher than those without TnT elevation and pre-existing CVD (69.44% vs 7.62%). In another study by Shi et al. (25), among 416 COVID-19 patients, 19.7% developed ACI. The mortality among patients with ACI was 11 times higher than those without (51.2% vs 4.5% p <0.001).
The exact mechanism of SARS-CovV-2 mediated ACI remains elusive, but there are several postulated mechanisms. Firstly, SARS-CoV-2 could cause down-regulation of ACE2 receptors in the heart and coronary vessels, which in turn triggers cytokine release, endothelial, myocardial inflammation or myocarditis (27, 28). Secondly, respiratory failure, hypoxia, hypoxemia secondary to COVID-19 pneumonia could cause ischemic damage to myocardium even in the absence of obstructive coronary artery lesions (27-29) .
Acute myocardial infarction (MI)
Mechanisms of MI in COVID-19 patients could be secondary to atherosclerotic plaque rupture and thrombotic occlusion (Type I MI). It could also be secondary to myocardial oxygen supply/demand mismatch (Type II MI) associated with hypoxia, hypoxemia, endothelial inflammation, microcirculation dysfunction, and cytokine storm. There was also case report of MI triggered by spontaneous coronary artery dissection in a COVID-19 patient (30). The clinical characteristics of COVID-19 patients presenting with STEMI were very different from those in typical STEMI patients who had atherosclerotic plaque rupture. Firstly, 21-67% of the patients did not complain of chest pain at presentation.
The clinical presentation of myocardial infarction in COVID-19 patients was often atypical and different from that in patients with atherosclerotic coronary artery disease.
Bangalore et al. (31) reported a series of 18 COVID-19 patients in New York, who presented with ST elevation in electrocardiogram (ECG). The median age of the patients was 63 years. Among the patients, 67% did not report any chest pain. Half of the patients underwent coronary angiography. Obstructive coronary artery disease was detected in only 67% of the patients (n = 6). Percutaneous coronary intervention (PCI) was performed in 5 of the 6 patients with obstructive coronary lesions. The in-hospital mortality was 50% and 90% among patients with confirmed MI and non-coronary myocardial injury respectively. Stefanini et al. (32) reported 28 COVID-19 patients with ST elevation myocardial infarction in Lombardy region in Italy. The mean age of the patients was 68 (11) years. Among these patients, 21% had no chest pain. All the patients had undergone coronary angiogram. Overall 61% of the patients had obstructive CAD and culprit lesions requiring coronary revascularization. The overall mortality of this cohort was 39%.
In Bangalore’s series (31), among patients with confirmed MI after coronary angiogram, 38% had no chest pain. Secondly, obstructive coronary artery lesions were only detected in 61-67% of patients who had undergone coronary angiogram. Thirdly, the mortality rate was very high, ranging from 39% to 72%. The mortality rate among patients without obstructive coronary artery lesion was even up to 90% (31). The atypical presentations highlighted the potential multifactorial pathophysiological mechanisms underlying the acute myocardial injury of STEMI in COVID-19 patients.
A)
B)
C)
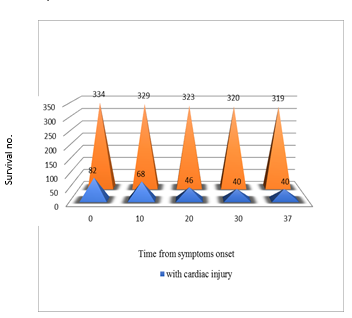
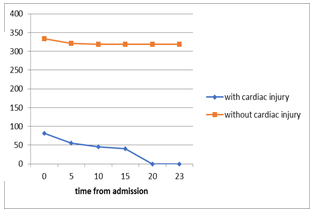
Figure 2. Guo et al reported a series of 187 hospitalized COVID-19 patients (26). (A) The mortality of COVID-19 patients with pre-existing cardiovascular disease (CVD) and elevated troponin T (TnT) was 9 times higher than those without pre-existing CVD and TnT elevation (69.44% vs 7.62%). The plasma TnT level of COVID-19 non-survivors showed escalating trend from admission to impending death. The plasma TnT level of COVID-19 survivors remained static throughout the course of hospitalization. Lower panel: Shi et al. reported a series of 416 COVID-19 patients. The mortality among patients with ACI was 11 times higher than those without acute cardiac injury (51.2% vs 4.5% p <0.001) (25). The Kaplan-Meier survival curves for mortality during the time from (B) symptom onset and (C) from admission showed that patients with cardiac injury have higher mortality.
In the world’s largest retrospective multicenter registry involving 109 high-volume primary percutaneous coronary intervention (PPCI) centers and 16 674 STEMI patients undergoing PPCI in March/June 2019 and 2020, from Europe, Latin America, South-East Asia and North Africa, the PPCI rate decreased by 16% during the pandemic period. There was a significant increase in door-to-balloon time (40 (25–70) vs 40 (25–64) min, p=0.01) and total ischemic time (225 (135–410) vs 196 (120–355) min, p<0.001) compared with the pre-pandemic period. The delay of coronary intervention among STEMI patients admitted in 2020 was associated with higher in-hospital (6.5% vs 5.3%, p<0.001) and 30-day (8% vs 6.5%, p=0.001) mortality observed during the pandemic, compared with the data in 2019 (33).
In the NACMI (North American COVID-19 STEMI) multicenter prospective observational registry, a total of 586 COVID-19–positive patients with STEMI were analyzed. Two hundred and twenty seven patients were treated in 2020 and 359 were treated in 2021. Compared with the patients in 2020, the patients admitted in 2021 had lower prevalence of shock before PCI (13% vs. 18%, p = 0.07) or pulmonary manifestations (33% vs. 47%, p = 0.001). The STEMI COVID-19 patients admitted in 2021 had lower in-hospital mortality 23% vs 33% in those admitted in 2020, p = 0.008. Vaccination was associated with lower mortality. None of the 22 vaccinated patients died in the hospital, while 22% of unvaccinated patients died in the hospitals (34).
Myocarditis
Patients with COVID-19 infection could also be complicated by myocarditis. The first case report of COVID-19 associated fulminant myocarditis was published in early March 2020 (35). In the case series of 150 Wuhan COVID-19 patients, 68 patients died. Among the deceased patients, 7% died of myocardial damage with circulatory failure. In 33% of the deceased patients, myocarditis might have played a contributing role to mortality (36).
Tavazzi et al (37) reported the first pathological evidence and myocardial localization of SARS-CoV-2 related myocarditis. The 69-year-old man with COVID-19 infection suffered from acute respiratory distress syndrome (ARDS) and cardiogenic shock requiring extracorporeal membrane oxygenation (ECMO). Endomyocardial biopsy showed low-grade interstitial and endocardial inflammation with vacuolated macrophages. Electron microscopy demonstrated viral inclusion bodies with protein spikes typical of corona virus within the myocardial interstitial cells. The patient died of septic shock.
In a German study of 100 patients recently recovered from COVID-19 infection (33% requiring hospitalization), cardiac magnetic resonance imaging (CMRI) (performed at a median of 71 days after diagnosis) revealed cardiac involvement in 78% of patients – including raised myocardial native T1, raised myocardial native T2, myocardial late gadolinium enhancement (LGE) or pericardial enhancement. Ongoing myocardial inflammation (defined as abnormal native T1 and T2 measures) was present in 60% of patients. Endomyocardial biopsy in 3 patients with severe CMRI findings revealed active lymphocytic inflammation with no evidence of any viral genome (38).
In a British study of 148 patients with severe COVID-19 infection (all requiring hospitalization and 32% required ventilatory support) and troponin elevation received CMRI at median of 68 days from diagnosis. LGE and/or ischemia was detected in 54% of patients (with myocarditis-like scar in 26%, infarction and/or ischemia in 22% and dual pathology in 6%. Among patients with myocarditis-like injury pattern, 30% (n=12) had CMRI evidence of active myocarditis while (68%) (n = 27) had findings consistent with healed myocarditis. On the other hand, a quarter of patients had ischemic heart disease of which 2/3 had no previous history. Myocardial infarction and inducible myocardial ischemia were detected in 19% and 26% of patients undergoing stress perfusion CMRI (39).
In a study from United States, 26 competitive college athletes with COVID-19 infection underwent CMRI after 11-53 days of quarantine. No athletes required hospitalization or antiviral therapy. No athletes had diagnostic electrocardiogram change or elevated serum troponin I. Four athletes (15%) had CMRI evidence consistent with myocarditis (myocardial oedema by elevated T2 signal and myocardial injury by presence of non-ischemic LGE). Twelve athletes (46%) had LGE (among whom 30.8% had no concomitant T2 signal elevation) (40).
In the COVIDsortium multicenter prospective study, 731 health care workers underwent weekly symptom, polymerase chain reaction and serology assessment over 4 months. Among the 731 health care workers, 21.5% had seroconversion (n=157). The symptoms were mild in 99%, with 25% being asymptomatic. Seventy-four of the seropositive patients and 75 seronegative control subjects were recruited. CMRI performed at 6 months showed similar cardiac structures, systolic function, global longitudinal strain and tissue characterization (T1 and T2 signals, extracellular volume and LGE) or biomarkers between seropositive and seronegative groups. The study concluded that CMRI abnormalities are no more common in seropositive healthy healthcare workers 6 months post mild COVID-19 infection (41).
A review of 277 autopsied hearts from 22 studies of COVID-19 patients demonstrated classic myocarditis, non-myocarditis inflammatory infiltrate, single-cell ischemia and acute myocardial infarction in 7.2%, 12.6%, 13.7% and 4.7% respectively (42). At least one cardiovascular histopathologic abnormality (e.g., macrovascular or microvascular thrombus, inflammation, or intraluminal megakaryocytes) was noted in 47.8% (42).
Post COVID-19 vaccine myocarditis
Messenger RNA (mRNA)-based COVID-19 vaccines induce spike-protein IgG antibodies to prevent the attachment of SARS-CoV-2 spike protein to the host cell angiotensin-converting enzyme 2 receptors. However, the mRNA-based COVID-19 vaccines may induce hyperimmunity and myocarditis through 3 potential mechanisms: mRNA immune reactivity, antibodies to SARS-CoV-2 spike protein cross-reacting with myocardial proteins, and hormonal signaling. (testosterone can inhibit anti-inflammatory immune cells and promote a more aggressive T helper 1 cell-type immune response) (43).
In a retrospective study, an increased risk of myocarditis or pericarditis was observed after COVID-19 mRNA vaccination and was highest in men aged 18–25 years after a second dose of the vaccine, with a pooled incidence rate of 1.71 per 100 000 person-days for BNT162b2 and 2.17 per 100 000 person-days for mRNA-1273 (44).
In a Israeli study of 2.5 million vaccinated healthcare organization members who had received at least one dose of the BNT162b2 mRNA vaccine, the estimated incidence of myocarditis was 2.13 cases per 100,000 persons. The highest incidence was among male patients between the ages of 16 and 29 years: 10.69 cases per 100,000. Most of the myocarditis cases were mild or moderate in severity. However, among 14 patients who had left ventricular dysfunction (LVD) during admission, 10 still had persistent LVD upon discharge (45).
In an United States study of 1626 cases of post COVID-19 vaccine myocarditis in a national reporting system, the rates of myocarditis cases were highest after the second vaccination dose in adolescent males aged 12 to 15 years (70.7 per million doses of the BNT162b2 vaccine), in adolescent males aged 16 to 17 years (105.9 per million doses of the BNT162b2 vaccine), and in young men aged 18 to 24 years (52.4 and 56.3 per million doses of the BNT162b2 vaccine and the mRNA-1273 vaccine, respectively) (46).
By the end of May 2021, the EudraVigilance database reported 122, 16, 38 and 0 cases of myocarditis after Comirnaty, Moderna, Vaxzevria and Janssen vaccines respectively in the European Economic Area (EEA). The exposure in the EEA for each vaccine was around 160 million doses for Comirnaty, 19 million doses for Moderna, 40 million for Vaxzevria and 2 million for Janssen (47).
By late May 2022, the Center for Disease Control estimated that a total of 491.9 million primary series and 1st booster mRNA COVID-19 vaccine doses have been administered in the Unites States among people ages 18 years and older. A total of 1321 verified cases of myocarditis after mRNA COVID-19 vaccination have been reported to VAERS. Among the 990 patients with known outcome, 73% recovered. There were 21 reported deaths involving myocarditis (48).
Despite the reported cases of COVID-19 vaccine related myocarditis, the CDC determined that the benefits of COVID-19 vaccination outweigh the risks of myocarditis (49).
Congestive heart failure
Cytokine release syndrome and systemic inflammatory response involving myocardium and endothelial cells in COVID-19, could trigger acute cardiac injury, myocarditis, MI, or Takotsubo cardiomyopathy (50), which could all in turn lead to the final common pathway of decompensated CHF. In the Wuhan series of 191 COVID-19 in-patients (11), 23% of the patient developed CHF. The prevalence of CHF among COVID-19 non-survivors was 4 times higher than that among the survivors (52% vs 12%).
In the global observational study of 8910 COVID-19 patients, 2% developed CHF. The in-patient mortality among those with CHF was 2.7 times higher than in those without (15.3% vs 5.6%). In Guo et al ‘s series of 187 COVID-19 patients, 27.8% suffered from myocardial injury. Plasma TnT level demonstrated positive and linear correlation with plasma N-terminal pro-brain natriuretic peptide (NT-proBNP) level ((β = 0.613, p<0.001). Escalating trend of NT-proBNP was observed throughout the course of hospitalization among COVID-19 non-survivors, but not among the survivors (26).
In the American Heart Association’s COVID-19 Cardiovascular Disease Registry, among the 8920 COVID-19 patients hospitalized between January 2020 to July 2020, 11% had history of heart failure (HF), which was associated substantially higher risk of mortality. In-hospital mortality occurred in 31.6% and 16.9% of patients with and without history of HF respectively (adjusted relative risk 1.16; 95% CI 1.03-1.3). History of HF with reduced ejection fraction was associated with even higher risk of mortality in COVID-19 patients (51).
In the national healthcare databases from the US Department of Veterans Affairs, the risk of incident cardiovascular disease beyond the first 30 days after COVID=19 infection was increased compared with historical control. Patient had elevated risk of HF (HR 1.72 (CI 1.65-1.8)) and non-ischemic cardiomyopathy (HR 1.62 (CI 1.52-1.73)) post COVID-19 infection (52).
In the Critical Care Cardiology Trials Network, among the 901 patients admitted to intensive care units (ICU) in 6 centers due to COVID-19, 8.9% had acute HF (including 18 patients with classic cardiogenic shock and 37 patients with vasodilatory cardiogenic shock). About 5% (45 patients) developed de novo HF. Among the critically ill COVID-19 patients, mortality rate was higher in those with heart failure versus those without (43.8% vs 32.4%; p=0.004) (53).
Cardiac arrhythmias
Palpitation was reported in about 7.3% of COVID-19 patients. In the cohort of 138 COVID-19 in-patients in Wuhan, 17% developed cardiac arrhythmia. The prevalence of cardiac arrhythmia was 44.4% among COVID-19 patients admitted to ICU (versus only 6.9% among those not admitted to ICU. COVID-19 patients with troponin elevation and ACI were more likely to develop malignant ventricular arrhythmia (MVA). The prevalence of MVA was 11.5% among COVID-19 patients with troponin elevation versus 5.2% in those without troponin elevation (11, 26, 54).
In-hospital cardiac arrest (IHCA)
In a study of 24 915 patients with IHCA from 286 hospitals, suspected or confirmed COVID-19 infection was present in 5916 patients (23.7%). Patients with COVID-19 had lower rates of survival to discharge (11.9% vs 23.5%; adjusted RR, 0.65 [95% CI, 0.60-0.71]; p<0.001) and restoration of spontaneous circulation (53.7% vs 63.6%; adjusted RR, 0.86 [95% CI, 0.83-0.90]; p<0.001). Compared with those without COVID-19 infection, patients with COVID-19 infection had higher risk of non-shockable rhythm - asystole (30.3% vs 25.6%) and pulseless electrical activity (60.2% vs 58.4%) (p<0.001) and lower risk of ventricular fibrillation or pulseless ventricular tachycardia (9.5% vs 15.9%) (p<0.001) (55).
Out-of-hospital cardiac arrest (OHCA)
In an Italian study, the incidence of OHCA increased by nearly 60% during the peak of 2020 COVID-19 pandemic (56). In a French study, the rate of OHCA increased by 52% during the pandemic period in 2020 (57).
In the French National OHCA registry, among the 6,624 OHCA patients, 127 and 473 patients had confirmed and suspected COVID-19 infection respectively. The "confirmed" and "suspected" COVID-19 patients were more likely to have suffered from respiratory causes of OHCA. The confirmed and suspected COVID-19 patients also received lower rate of advanced life support was (57.5% and 64.5% respectively, p=0.002) versus those without COVID-19 infection. Among the confirmed COVID-19 patients, none survived beyond 30 days post OHCA, versus 0.9% and 3.5% (p=0.023) in those with suspected COVID-19 infection and those without COVID-19 infection respectively (58).
In a United States study of 19 303 OHCA patients between 2019 and 2020, the rates of ROCS during the pandemic were lower than that in 2019 (23.0% vs 29.8%; adjusted rate ratio, 0.82 [95% CI, 0.78-0.87]; p<0.001) in communities with high or very high COVID-19 mortality, respectively; however, rates of sustained ROSC were also lower by 11 – 15%. The survival to discharge was lower during the pandemic compared with that in 2019 (6.6% vs 9.8%; adjusted RR, 0.83 [95% CI, 0.69-1.00]; p=0.048) (59).
In the worldwide survey of COVID-19 associated arrhythmias, among the 4526 COVID-19 patients from 4 continents and 12 countries, 827 (18%) developed cardiac arrhythmia. Hypertension, diabetes, HF and CAD were present in 69%, 42%, 30% and 24% of patients. Among patients with cardiac arrhythmia, 81.8% developed atrial arrhythmias, while 20.7% 22.6% developed ventricular arrhythmias, and bradyarrhythmia respectively. Asian patients had lower incidence of atrial fibrillation (AF) (34% vs 63%). Only half of the cardiac arrhythmia COVID-19 patients (51%) survived to hospital discharge (60).
Pharmacological therapies for COVID-19 infection including lopinavir/ritonavir, azithromycin (AZT), chloroquine and hydroxychloroquine (HCQ) could block KCNH2-encoded HERG/Kv11.1 delayed rectifier outward potassium channel and cause QT prolongation.
Specific cardiac arrhythmias
QTc prolongation
In a study of 415 COVID-19 patients who received concomitant hydroxychloroquine and azithromycin, the mean QTc increased from 443 (25) ms to a maximum of 473 (40) ms (87 [21%] patients had a QTc ≥500 ms). No significant ventricular arrhythmia or increase in mortality were observed in patients with drug induced QTc prolongation (61).
In a multicenter study of 2014 COVID-19 patients treated with hydroxychloroquine alone or in combination with azithromycin, 1.7-3.3% developed severe QTc prolongation ≥500 ms. No patient developed polymorphic ventricular arrhythmia or arrhythmic death. Combination therapy was associated with more pronounced QTc prolongation versus hydroxychloroquine therapy alone (22.2 ms vs 11.0 ms, p<0.001) (459.8 (21.4) ms vs 438.4 (29.9) ms, p<0.001) (62).
Ventricular arrhythmia
In a study of 143 COVID-19 patients admitted with telemetry monitoring, premature ventricular complexes (PVC) and non-sustained ventricular tachycardia (NSVT) occurred in 28.7% and 15.4% of patients respectively. There was no significant difference in the prevalence of PVC and NSVT between survivors and non-survivors. Sustained ventricular tachycardia and ventricular fibrillation were detected in 1.4% and 0.7% of patients, respectively (63).
Atrial fibrillation
In a large-scale United States registry of 30999 hospitalized COVID-19 patients, 5.4% developed new-onset AF during index hospitalization. New-onset AF was associated with higher risk of death (45.2% versus 11.9%) and major adverse cardiac events (23.8% versus 6.5%) (64). In a meta-analysis with 21653 hospitalized COVID-19 patients, the prevalence of AF was 11%. New-onset AF was associated ed with an increased risk of all-cause mortality among patients with COVID-19 ((OR: 2.32, 95% CI: 1.60 to 3.37) (65).
Bradyarrhythmia
In the Worldwide Survey of COVID-19-Associated Arrhythmias, among the 4526 COVID-19 patients, 172 patients developed bradyarrhythmia - including bradycardia (12.8%), atrioventricular block (8.6%), or a pause greater than 3 seconds (1.2%) (60).
Channelopathies
The use of QT prolonging anti-SARS-CoV2 medications could potentially increase the risk of ventricular arrhythmia in patients with congenital long QT syndrome. Fever during COVID-19 infection could also unmask cardiac channelopathies such as Brugada syndrome and long QT syndrome and potentially increase the risk of ventricular arrhythmia (66, 67).
Conclusion
Corona virus 2019 (COVID-19) is caused by SARS-CoV-2. The SARS-CoV-2’s surface spike protein binds to ACE2 receptors for host cells entry and viral replication. Patients with pre-existing CVD have upregulation of ACE2, which are expressed in cardiomyocytes and vascular endothelial cells. The ACE2 upregulation in patients with CVD could promote SARS-CoV-2 viral infection. The SARS-CoV-2 could cause disruption and downregulation of the cardioprotective ACE2, triggering cytokine release, cardiovascular system
and systemic inflammation. This pathophysiological mechanism could explain the high prevalence of CVD
and cardiovascular complications in COVID-19 patients. Cardiovascular complications in COVID-19 include acute cardiac injury, myocarditis, cardiomyopathy, myocardial infarction, coronary artery dissection, cardiac arrhythmia, congestive heart failure, pulmonary embolism, circulatory collapse and cardiogenic shock. The presence of the above cardiovascular complications is associated poor prognosis and high mortality. Pharmacological therapies for COVID-19 could cause QT prolongation and trigger Torsades de Pointes, especially in patients with underlying channelopathies. COVID-19 vaccines have been shown to be associated with a small risk of myocarditis especially in young men. However, the overall benefit of vaccination outweighs its absolute risk. The rare occurrence of COVID-19 vaccine related complications should not deter patients from receiving timely vaccination.
Peer-review: internal
Conflict of interest: None to declare
Authorship: J.K.C., O.A., E.G., Sh.M., and N.K. equally contributed to the preparation of manuscript
Acknowledgement and funding: None to declare
References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

Rötteln Castle, popularly known as "Röttler Castle", is located in the extreme south-west of Baden-Württemberg, in the border triangle of Switzerland, France and Germany. It is one of the most imposing medieval fortresses and is the third largest castle ruin in Baden. Photography by Martha Klinckwort, Basel, Switzerland
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

