Procalcitonin as a predictor of survival in patients with sepsis: A study on diagnostic accuracy
ORIGINAL RESEARCH ARTICLE
Procalcitonin as a predictor of survival in patients with sepsis: A study on diagnostic accuracy
Article Summary
- DOI: 10.24969/hvt.2024.454
- CARDIOVASCULAR DISEASES
- Published: 17/01/2024
- Received: 23/09/2023
- Revised: 09/12/2023
- Accepted: 09/01/2024
- Views: 6711
- Downloads: 3315
- Keywords: sepsis, systemic inflammatory response syndrome, procalcitonin, survival, outcome, diagnostic accuracy
Address for Correspondence*: Abhishek Verma, Department of Cardiac Anesthesia, U. N. Mehta Institute of Cardiology and Research Center, Civil Hospital Campus, Asarwa, Ahmedabad-380016, Gujarat, India
Abhishek Verma1*, Karan Kaushik1, Amit Taluja1, Pratik Shah2
1Department of Cardiac Anesthesia, U.N.Mehta Institute of Cardiology and Research Center Ahmadabad, Gujarat, India
1U.N.Mehta Institute of Cardiology and Research Center Ahmadabad, Gujarat, India
Abstract
Objective: Role of serum procalcitonin (PCT) in critically ill patients is well defined for identification of bacterial infection but it’s role in prediction of survival is not well established. We studied plasma kinetics of PCT, over the first three critical care days to validate its role in prediction of survival of patients with sepsis according to plasma level.
Methods: A prospective observational study was conducted in CCU (KGMU). Total 53 consecutive patients either sex were recruited. Patients with systemic inflammatory response syndrome (SIRS) were recruited. PCT was measured on 1st and 3rd days. We studied 50 days survival using Kaplan-Meier analysis and diagnostic accuracy of PCT using ROC analysis.
Results: In our study, PCT was statistically significantly higher in non-survivor patients compared to survivor patients (p<0.05). ROC curve was made with PCT based on culture at both time intervals. On the basis of ROC curve, we determined a cut-off value as 12.21 ng/ml of PCT on day 3 to predict sepsis with high sensitivity (93.7%) and specificity (71.4%). Area under curve (AUC) was also higher observed at day 3 (AUC=0.864, p<0.001) compared to day 1 (AUC=0.658, p<0.05). According to Kaplan-Meier curve, risk of mortality was by 83% (HR 0.17(95% 0.07-0.44)) lower in low sepsis category group (≤12.21 ng/ml) compared to high sepsis category group (>12.21 ng/ml).
Conclusion: According to our study results, we conclude that serum PCT has good clinical diagnostic and prognostic values in prediction of survival in patients with sepsis. Kinetic studies of PCT can improve sensitivity and accuracy when evaluating the prognosis of patients with sepsis as well as survival rate.
Key words: sepsis, systemic inflammatory response syndrome, procalcitonin, survival, outcome, diagnostic accuracy
Introduction
An infection-induced combination of physiologic, pathologic, and biochemical abnormalities known as sepsis is a serious public health concern. In 2011, the US hospital industry spent over $20 billion (5.2%) of its overall costs. Sepsis incidence reports are rising, probably due to ageing populations with more comorbidities, increased awareness, and, in certain cases, coding that is more lenient on reimbursements. Sepsis is a major cause of death and critical disease globally, however the real prevalence is unknown and estimates are conservative (1).
Some inflammatory markers include C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white blood cell count (WBC); however, their usefulness in bacterial infection is limited due to their poor sensitivity and specificity. Furthermore, although microbiological cultures are the gold standard for diagnosing sepsis, their results can occasionally be confused with false positive or false negative results since they do not reflect the host's reaction to inflammation. Due to these shortcomings in existing blood tests and culture, researchers have discovered additional markers that are more precise and sensitive. Particular attention has recently been paid to procalcitonin (PCT) as a distinct and early indicator of sepsis and systemic inflammation in both adults and children (2).
Hyperprocalcitoninemia is brought on by systemic inflammation or infection and develops in 2-4 hours, peaks in 8-24 hours, and lasts as long as the inflammatory process lasts. Since PCT has a half-life of roughly 24 hours, concentrations return to normal as soon as the patient recovers.
Furthermore, a number of studies have demonstrated that the consistent application of PCT for sepsis positively affects the decrease in antibiotic treatment, hence enabling a shorter intensive care unit (ICU) stay and lowering case expenses (3, 4).
Therefore, the aim of the study was evaluate the diagnostic accuracy of PCT as a biomarker in the ICU unit to predict survival in patients with sepsis at our tertiary care center.
Methods
This was a prospective observational study conducted in the critical care unit at King George Medical University, after approval from the institute’s Ethical committee. The study was conducted over the period of 1 year (June 2016 – June 2017). We enrolled 53 patients based on inclusion as well as exclusion criteria. These patients were divided into two groups: survivors and non-survivors. Enrolled patients provided inform consent.
A total of 53 patients of either sex in the age group of 12-85 years, fulfilled the criteria for sepsis (inclusion criteria) as followings:
Inclusion Criteria
The clinical parameters include two or more the following: Fever (>38° C) or hypothermia (<36° C), increased heart rate (>90 beats/min), tachypnea (>20 breaths/min) or hyperventilation (PaCO2 < 32 mmHg), and altered white blood cell count (>12,000 cells/mm3 or <4000 cells/mm3) or presence of >10% immature neutrophils.
Exclusion Criteria: (1) patient not giving consent (2) Post cardiopulmonary resuscitation patients (3) Patients referred from other intensive care unit and (4) liver disease
Baseline variables
A detailed history, demohraphic, clinical and laboratory (including WBC, hemoglobin, CRP and PCT findings, and complications were noted.
Sample collection and measurement of PCT
Sample for Procalcitonin was collected at the time of admission (day 1) and at day 3.
PCT calculation
We determined PCT via Elecsys BRAHMS PCT assay for early detection of sepsis.
Test principle: Sandwich principle. Total duration of assay: 18 minutes
1st incubation: Antigen in the sample (18 µL), a biotinylated monoclonal PCT specific antibody, and a monoclonal PCT specific antibody labeled with a ruthenium complex {Tris(2,2'-bipyridyl) ruthenium(II)-complex (Ru(bpy))} react to form a sandwich complex.
2nd incubation: After addition of streptavidin-coated microparticles, the complex becomes bound to the solid phase via interaction of biotin and streptavidin.
The reaction mixture was aspirated into the measuring cell where the microparticles were magnetically captured onto the surface of the electrode. Unbound substances were then removed with ProCell/ProCell M. Application of a voltage to the electrode then induces chemiluminescent emission which was measured by a photomultiplier.
Results were determined via a calibration curve which was specifically generated by 2 point calibration and a master curve provided via the reagent barcode.
Measuring range of Elecsys BRAHMS PCT assay is 0.02 - 100 ng/mL (defined by the limit of detection and the maximum of the master curve).
Statistical analysis
Statistical analysis was done by SPSS 26.0 version (IBM SPSS .inc, Chicago). Categorical data are represented as number with percentage whereas continuous data are represented as mean with standard deviation. Chi- square test was used for categorical variables comparison test. Mann-Whitney test was used for non-parametric data analysis. To predict a cut off value for diagnosis, we were used ROC curve analysis. Cox proportional regression analysis was useful to predict hazard risk (HR) of mortality based on PCT categories and Kaplan-Meier survival analysis (Log Rank test) was used to estimate survival rate. A p<0.05 value considered as a significant value for analysis.
Results
We were performed a prospective observational study between June 2016 to June 2017 at our institute. For this study, we were enrolled 53 patients during this study period based on inclusion and exclusion criteria of our study design. Among them 60.38% were non-survivors whereas 39.62% were survivors.
Overall mean age of the survivor and non-survivor patients was respectively as 49.43 (21.85) years and 39.69 (15.38) years. Among non-survivor patients, 79.17% were females whereas 44.83% were was males, and the difference was statistically significant (p=0.011).
We carried out laboratory investigations among 53 patients at day 1 and day 3, including followings parameters: Hb, WBC, CRP and PCT. We were did observed significant differences in HB levels between survivor and non-survivor patients at day 1 (p=0.1370) as well as day 3 (p=0.7920).
ROC curve
Based on sepsis culture, we performed ROC curve analysis with PCT as well as CRP at both interval s (Day 1 and Day 3). Using ROC curve, we evaluated sensitivity and specificity of PCT (day 1) and PCT (day 3) as followings: (93.7%, 47.6%), (93.7%, 71.4%). We observed that specificity also increased during day 3 to day 1 period. In this study, a cut off value for PCT at day 1 and day 3 respectively was ≤ 20.8 ng/ml and ≤ 12.21 ng/ml. The ROC curve analysis showed significant diagnostic value of PCT in prediction og sepsis complications at both intervals (day 1 and day 3) but the highest AUC was at day 3 (AUC=0.864, p<0.001) followed by day 1 (AUC=0.658, p<0.05). (Fig. 1, 2).
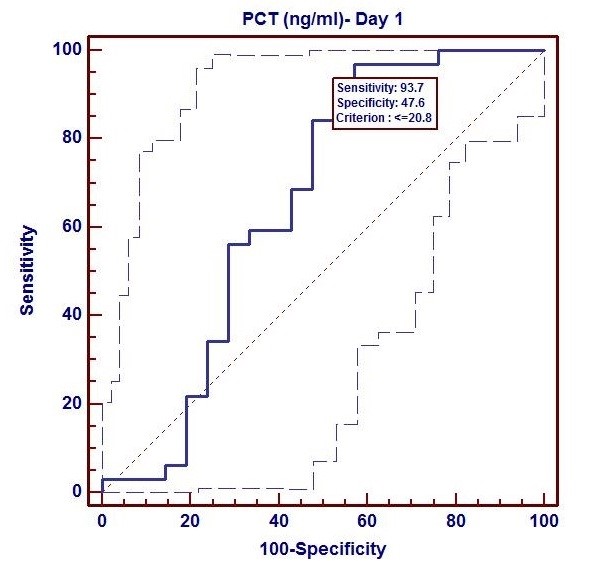
Figure 1. ROC Curve of procalcitonin at day 1 (AUC=0.864, p<0.001)
AUC – area under the curve, PCT- procalcitonin
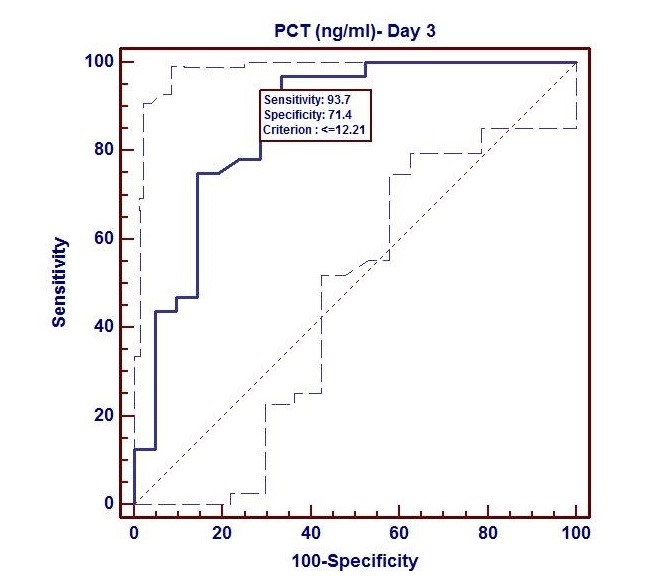
Figure 2. ROC Curve of procalcitonin at day 3 (AUC=0.658, p<0.05)
AUC – area under the curve, PCT- procalcitonin
Survival analysis
In this study, we performed Kaplan-Meier survival curve analysis based on mortality with high and low PCT values at both time intervals (Day1 and Day3). We also used cut-off value, which evaluated based on ROC curve analysis at both time periods. Above the cutoff value was considered as high category PCT whereas below the cutoff value considered as low category. Cox proportional regression analysis was useful to predict hazard risk (HR) of mortality based on PCT categories. In this study, we observed that high category PCT levels statistically significantly predicted mortality at both intervals: day 1 (Log rank test χ2 = 13.69; HR (95%CI) = 0.11 (0.4-0.36) and day 3 (Log rank test χ2 =13.81; HR (95%CI) = 0.17 (0.07-0.44) (Fig. 3, 4).
Discussion
In order to identify and evaluate disease prognosis, research efforts are currently concentrated on PCT-level kinetics and CRP-level kinetics (3, 4). The body maintains a relative equilibrium with very low levels of serum PCT and CRP under normal physiological conditions. The host's reaction to the infection results in a significant rise in serum PCT and CRP levels when sepsis is brought on by an inflammatory stimulus induced by a bacterial infection. In sepsis patients, PCT is a useful biochemical measure of the extent of infection. Sepsis or septic shock are indicated by a PCT of 2 ng/mL (5-7). CRP levels can rise more than 100 times over baseline levels, signifying an ongoing illness.
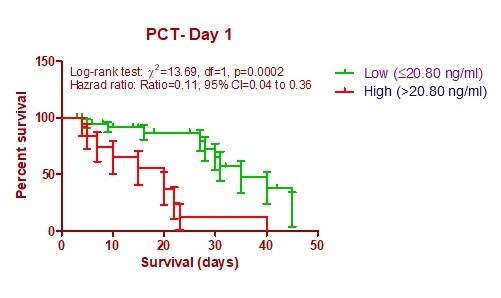
Figure 3. Kaplan-Meier survival curves based on PCT levels at day 1
PCT- procalcitonin
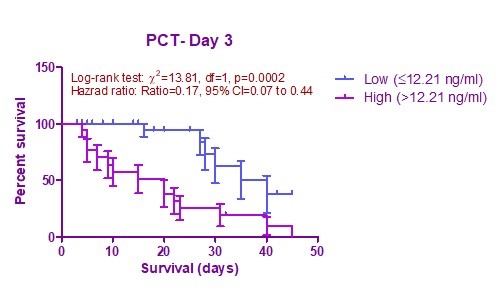
Figure 4. Kaplan-Meier survival curves based on PCT levels at day 3
PCT- procalcitonin
It has been observed that despite the use of optimal support and better strategy, the mortality rate in sepsis still remains high. To diagnose sepsis early in addition to clinical examination, various strategies are used in ICU (APACHE II and SOFA scores, sepsis markers, culture and sensitivity tests etc.).
Our study showed that sepsis marker could be the better choice in some overlapping or masked conditions.
A study done by Hamade and Huang in 2019 describes a conceptual framework of biomarker using lesson from history of troponin and use this concept to procalcitonin (8).
Physiology of procalcitonin
A propeptide of calcitonin with no hormone activity is called procalcitonin, a glycoprotein. Under normal conditions, the thyroid gland's C-cells synthesize this 116 amino acid protein, which has a molecular weight of 13 kD (9, 10). A particular protease then cleaves procalcitonin, yielding calcitonin, katacalcin, and an N-terminal residue (9). Procalcitonin has a lengthy half-life of 25–30 hours in serum (11), compared to the short half-life of calcitonin (10 min). Procalcitonin levels in healthy people are negligible (<0.1 ng/ml). Procalcitonin, which is largely produced by extra-thyroid tissues, can reach levels of above 100 ng/ml during severe infections (bacterial, parasitic, and fungal) with systemic symptoms. Therefore, during a severe viral episode (12), people who have previously undergone a total thyroidectomy can still produce significant quantities of procalcitonin. The exact site of procalcitonin production during sepsis is uncertain; one investigator, using katacalcin antibodies, has identified procalcitonin-like activity in human leukocytes, (13) others suggest neuroendocrine cells and the lungs (14,15) as possible sites of production. Remarkably, the large amounts of procalcitonin produced during infections do not lead to an increase in plasma calcitonin levels or activity (12).
Many studies have validated the use of PCT for risk stratification in the ICU and the emergency department. This study was conducted to investigate the potential role of PCT as early predictors of survival in sepsis.
Role of PCT in survival
Positive correlation of inflammatory cytokines with PCT and its role in survival was proved in past studies. Meisner et al. (13) found that PCT of non-surviving patients was initially not different from that of survivors but significantly increased after the fourth day following onset of the disease. Similarly, in a study of Seligman et al. (14), it was concluded that measurement of PCT and CRP at onset and the fourth day of treatment can predict the survival.
Karlsson et al. (16) showed that PCT concentrations did not differ between hospital survivors and non-survivors but mortality was lower in patients whose PCT concentration decreased > 50% (by 72 hours) compared to those with a < 50% decrease in PCT. Schuetz et al. (15), suggested that monitoring PCT kinetics in the first 72 hours of critical care provides information that may potentially help, early transfer and therapy intensification decisions. Mat et al. (17) in 2014 demonstrated that serum PCT measured within 24 hours of ICU admission was associated with diagnosis of sepsis; however, it was not predictive of mortality but dynamic changes in PCT at 48 hours (PCT clearance) predicted survival.
A meta-analysis done by Tan M et al. in 2019 (18), evaluated role of CRP and procalcitonin in sepsis and they concluded that diagnostic accuracy of PCT was higher than CRP`s (18). In same year Song et al. (19) undertook a prospective controlled study to compare prognostic role of interleukin-6 (IL-6), pentraxin and PCT in sepsis. Their study showed that IL-6 was superior to penteaxin-3 and PCT (19). As IL-6 is not readily available everywhere, and also costly so treatment cost will have increased, in contrast PCT, it easily available and cost-effective. Cong et al. in 2021 (20) conducted a meta-analysis to evaluate accuracy of neutrophil CD64, PCT and IL-6 for diagnosis of sepsis. They concluded in their study that neutrophil CD26 had highest diagnostic value followed by PCT and IL-6. PCT had better diagnostic potential for diagnosis of sepsis in patients with severe condition compared with non-severe condition (20). Westwood M et al. (21), performed a systemic review for PCT- guided antibiotic therapy and cost effective analysis and they found PCT- guided treatment had a probability of >84% of being cost effective in their setting.
Nylen et al. (22) denoted that PCT, being an important marker of severity of systemic inflammation and mortality, also is an integral part of the inflammatory process and directly affects the outcome. Similarly, Luzzani et al. (23) also shows that course of PCT shows a closer correlation with the severity of infection and organ dysfunction.
Na et al. (24) observed PCT level and CRP level during four intervals at (Day 1, Day 2 Day 3 and Day 5). At all the intervals, he found that non-survivor patients had PCT values significantly higher compared to survivor patients. Our study findings are similar to the findings of this study.
In our study, area under ROC curve was used to predict sepsis outcome with the help of PCT at day 1 (AUC=0.827, p<0.001), day 3 (AUC=0.864, p<0.001) and observed between difference of day 1 and day 3. Area under curve based cutoff value observed during day 1 and day 3 as followings: ≤20.8 ng/ml and ≤12.21 ng/ml.
During day 3 we observed higher area under curve value compared to day 1. Our study results are in agreement with the study results reported by Rey et al. (25). He found median plasma concentration during systemic inflammatory response syndrome (SIRS), localized infection, sepsis, severe sepsis, and septic shock groups followings respectively as: 0.17 ng/ml ,0.43 ng/ml,0.79 ng/ml, 1.80 ng/ml, 15.00 ng/ml and 19.13 ng/ml. We observed higher sensitivity and specificity level at day 3 compared to day 1.
We used cutoff value from ROC curve in survival prediction by Kaplan-Meier analysis. We found that PCT at day 3 [Log rank testχ2 = 13.69; HR (95%CI) = 0.11 (0.4-0.36), p= 0.0002]predicts better survival compared to day 1 [Log rank test χ2 = 13.81; HR (95%CI) = 0.17 (0.07-0.44), p=0.0002]. In our study, PCT value (>12.21ng/ml) at 72 hours had higher mortality rate compared to PCT value (≤ 12.21 ng/ml). Cui Na et al. (24) also found similar study results with our study findings. Another study of Hegazy et al. (26) revealed that a group of patients with PCT value of >19ng/ml had higher mortality compared to another group.
Study limitations
This study has several limitations. The number of patients included in the study is small and our study is also a single -center study. A multi-center study involving larger patient population may confirm our findings. Some patients might have had malignancy or surgical complications, such as acute liver failure after hepatectomy, so in these patients bacteremia was not cause of death. To assess the value of PCT for predicting survival in severe bacteremia, it might be acceptable to consider sepsis-related mortality.
Conclusion
Early recognition and the start of antibiotic treatment, fluid resuscitation, source control, and close patient monitoring remain the cornerstone of care to lower sepsis related morbidity and mortality. PCT has a good diagnostic and prognostic value in prediction of mortality in sepsis patients. Instead of looking PCT value at day 1, day 3 PCT value increases sensitivity for diagnosis of sepsis related mortality and better predicts survival. Therefore, before culture arrived we can easily have diagnosed complications of sepsis and save our patients.
Ethics: Informed consent was obtained from patients before all procedures and study protocol was approved by institutional Ethics Committee
Peer-review: External and internal
Conflicts of interest: None to declare
Authorship: A.V., K.K., A.T., and P.S. equally contributed to study and manuscript preparation.
Acknowledgement and funding: None to declare
References
| 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801-10. https://doi.org/10.1001/jama.2016.0287 PMid:26903338 PMCid:PMC4968574 |
||||
| 2.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003; 31: 1250-6. https://doi.org/10.1097/01.CCM.0000050454.01978.3B PMid:12682500 |
||||
| 3.Li Q, Gong X. Clinical significance of the detection of procalcitonin and C-reactive protein in the intensive care unit. Exp Ther Med 2018; 15: 4265-70. https://doi.org/10.3892/etm.2018.5960 PMid:29731821 PMCid:PMC5921035 |
||||
| 4.Demir NA, Sumer S, Celik G, Afsar RE, Demir LS, Ural O. How should procalcitonin and C‐reactive protein levels be interpreted in haemodialysis patients? Intern Med J 2018; 48: 1222-8. https://doi.org/10.1111/imj.13952 PMid:29717808 |
||||
| 5.Kade G, Literacki S, Rzeszotarska A, Niemczyk S, Lubas A. Removal of procalcitonin and selected cytokines during continuous veno-venous hemodialysis using high cut-off hemofilters in patients with sepsis and acute kidney injury. Blood Purif 2018; 46: 153-9. https://doi.org/10.1159/000488929 PMid:29705804 PMCid:PMC6008874 |
||||
| 6.Charles MV, Kalaivani R, Venkatesh S, Kali A, Seetha KS. Evaluation of procalcitonin as a diagnostic marker in neonatal sepsis. Indian J Pathol Microbiol 2018; 61: 81-4. https://doi.org/10.4103/IJPM.IJPM_820_16 PMid:29567889 |
||||
| 7.Wu CC, Lan HM, Han ST, Chaou CH, Yeh CF, Liu SH, et al. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: a systematic review and meta-analysis. Ann Intens Care 2017; 7: 1-6. https://doi.org/10.1186/s13613-017-0316-z PMid:28875483 PMCid:PMC5585118 |
||||
| 8.Hamade B, Huang DT. Procalcitonin: where are we now? Crit Care Clin 2020; 36: 23-40. https://doi.org/10.1016/j.ccc.2019.08.003 PMid:31733680 PMCid:PMC6866676 |
||||
| 9.Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 2002; 323: 17-29. https://doi.org/10.1016/S0009-8981(02)00101-8 PMid:12135804 |
||||
| 10.Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med 2008; 36: 941-52. https://doi.org/10.1097/CCM.0B013E318165BABB PMid:18431284 |
||||
| 11.Chua AP, Lee KH. Procalcitonin in severe acute respiratory syndrome (SARS). J Infect 2004; 48: 303-6. https://doi.org/10.1016/j.jinf.2004.01.015 PMid:15066330 PMCid:PMC7133698 |
||||
| 12.Oberhoffer M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med 1999; 134: 49-55. https://doi.org/10.1016/S0022-2143(99)90053-7 PMid:10402059 |
||||
| 13.Meisner M, Tschaikowsky K, Palmaers T, Schmidt J. Comparison of procalcitonin (PCT) and C-reactive protein (CRP) plasma concentrations at different SOFA scores during the course of sepsis and MODS. Crit Care 1999; 3: 1-6. https://doi.org/10.1186/cc306 https://doi.org/10.1186/cc309 PMid:11094475 PMCid:PMC137225 |
||||
| 14.Seligman R, Meisner M, Lisboa TC, Hertz FT, Filippin TB, Fachel JM, et al. Decreases in procalcitonin and C-reactive protein are strong predictors of survival in ventilator-associated pneumonia. Crit Care 2006 ;10: 1-9. https://doi.org/10.1186/cc3910 https://doi.org/10.1186/cc4348 https://doi.org/10.1186/cc5036 https://doi.org/10.1186/cc4828 https://doi.org/10.1186/cc4927 PMid:16934135 PMCid:PMC3226135 |
||||
| 15.Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care 2013; 17: 1-8. https://doi.org/10.1186/cc12787 PMid:23787145 PMCid:PMC4057444 |
||||
| 16.Karlsson S, Heikkinen M, Pettilä V, Alila S, Väisänen S, Pulkki K, et al. Predictive value of procalcitonin decrease in patients with severe sepsis: a prospective observational study. Crit Care 2010; 14: 1-0. https://doi.org/10.1186/cc9327 PMid:21078153 PMCid:PMC3219988 |
||||
| 17. Mat Nor MB, Ralib A. Procalcitonin clearance for early prediction of survival in critically ill patients with severe sepsis. Crit Care Res Pract 2014; 2014819034: https://doi.org/10.1155/2014/819034 PMid:24719759 PMCid:PMC3955692 |
||||
| 18.Tan M, Lu Y, Jiang H, Zhang L. The diagnostic accuracy of procalcitonin and C‐reactive protein for sepsis: A systematic review and meta‐analysis. J Cell Biochem 2019; 120: 5852-9. https://doi.org/10.1002/jcb.27870 PMid:30417415 |
||||
| 19.Song J, Park DW, Moon S, Cho HJ, Park JH, Seok H, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis 2019; 19: 1-1. https://doi.org/10.1186/s12879-019-4618-7 PMid:31718563 PMCid:PMC6852730 |
||||
| 20.Cong S, Ma T, Di X, Tian C, Zhao M, Wang K. Diagnostic value of neutrophil CD64, procalcitonin, and interleukin-6 in sepsis: a meta-analysis. BMC Infect Dis 2021; 21: 1-7. https://doi.org/10.1186/s12879-021-06064-0 PMid:33902476 PMCid:PMC8072745 |
||||
| 21.Westwood M, Ramaekers B, Whiting P, Tomini F, Joore M, Armstrong N, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technology Assessment 2015; 19: 3-236. https://doi.org/10.3310/hta19960 PMid:26569153 PMCid:PMC4781547 |
||||
| 22.Nylen ES, Whang KT, Snider RH, Steinwald PM, White JC, Becker KL. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit. Care Med 1998 ;26: 1001-6. https://doi.org/10.1097/00003246-199806000-00015 PMid:9635646 |
||||
| 23.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med 2003; 31: 1737-41. https://doi.org/10.1097/01.CCM.0000063440.19188.ED PMid:12794413 |
||||
| 24.Cui N, Zhang H, Chen Z, Yu Z. Prognostic significance of PCT and CRP evaluation for adult ICU patients with sepsis and septic shock: retrospective analysis of 59 cases. J Int Med Res 2019; 47: 1573-9. https://doi.org/10.1177/0300060518822404 PMid:30656987 PMCid:PMC6460616 |
||||
| 25.Rey C, Los Arcos M, Concha A, Medina A, Prieto S, Martinez P, et al. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Inten Care Med 2007; 33: 477-84. https://doi.org/10.1007/s00134-006-0509-7 https://doi.org/10.1007/s00134-007-0650-y |
||||
| 26.Hegazy MA, Omar AS, Samir N, Moharram A, Weber S, Radwan WA. Amalgamation of procalcitonin, C-reactive protein, and sequential organ failure scoring system in predicting sepsis survival. Anesth Essays Res 2014; 8: 296. https://doi.org/10.4103/0259-1162.143115 PMid:25886324 PMCid:PMC4258970 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

