The effect of an isolated fat diet on the blood vessels of the rat´s brain (experimental randomized study)
ORIGINAL RESEARCH ARTICLE
The effect of an isolated fat diet on the blood vessels of the rat´s brain (experimental randomized study)
Article Summary
- DOI: 10.24969/hvt.2024.527
- CARDIOVASCULAR DISEASES
- Published: 02/12/2024
- Received: 23/09/2024
- Revised: 14/11/2024
- Accepted: 15/12/2024
- Views: 3544
- Downloads: 2189
- Keywords: High-fat diet, rats, blood vessels, brain, microvascular circulation, lactate
Address for Correspondence: Elmira M Mamytova, Department of Neurology and Clinical Genetics named after A.M. Murzaliev, Kyrgyz State Medical Academy named after I.K. Akhunbaev, Bishkek, Kyrgyzstan
Email: elmiramamytova@yahoo.com Mobile: +996551325314
ORCID: Altynbekova Aigul - 0009-0000-8929-6929; Elmira M. Mamytova - 0000-0002-4322-5555; Yusuf Kh-M Shidakov - 0000-0002-2779-5574; Aycholpon T. Israilova -0009-0004-7373-5814; Alymjan ulu Bolotbek-0000-0001-9082-7279; Rustam R. Tuhvatshin- 0000-0002-9329-8568
Aigul T. Altynbekova1, Elmira M. Mamytova2a, Yusuf Kh-M Shidakov3, Aicholpon T. Israilova2a,3, Bolotbek Alymjan ulu2a, Rustam R. Tuhvatshin2b
1NAO "National Center for Child Rehabilitation", Astana, Republic of Kazakhstan
2aDepartment of Neurology and Clinical Genetics, and 2bDepartment of Pathological Physiology, Kyrgyz State Medical Academy named after I. K. Akhunbayev, Bishkek, Kyrgyz Republic
3Kyrgyz-Russian Slavic University named after B. N. Yeltsin, Bishkek, Kyrgyz Republic
Objective: Epidemiological studies show that recently there has been an increase in the number of cerebrovascular diseases and the mortality rate among the population. This trend is associated with a changing of metabolic processes in the body, in particular lipid metabolism, caused by an unbalanced high-calorie diet.
The aim of the study was to investigate the effect of an isolated fat diet on lactate blood level and histomorphological changes in microcirculation of the rats` brain.
Methods: The design of the study is experimental randomized. The study was conducted on sexually mature 7-8 months old lineal Wistar male rats, which were randomly divided into 2 groups – control (n=10) and experimental (n=9). The rats of the experimental group 1 ("isolated fat diet") were fed a diet consisting of fat-tailed sheep for 30 days. The control group was on a standard vivarium diet. Animal weight gain, serum lactate levels and histomorphological changes in the blood vessels of the brain regions of experimental animals were observed. Statistical processing of the results was carried out using the t-test for independent and paired samples.
Results: No increase in total weight was observed in the animals of the experimental group, on the contrary, weight loss in rats was noted (p<0.001). During the experiment, a 2.1-fold increase in lactate levels was verified in animals of the experimental group (p<0.001). An unbalanced diet led to a number of pathological changes of the structural integrity of the wall of the microvessels of the rat brain, which promoted a slowdown in blood flow and blood cells aggregation and, ultimately lead to the development of blood clots.
Conclusion: The obtained experimental data show changes in lipid metabolism and vascular remodeling of the microcirculatory bed of the rat brain due to increased fat intake.
Keywords: High-fat diet, rats, blood vessels, brain, microvascular circulation, lactate.
Introduction
Diets composed of high saturated fat and cholesterol cause damage to peripheral organs such as the heart (i.e. cardiovascular disease) (1), pancreas (i.e. type 2 diabetes mellitus) (2) and liver (i.e. fatty liver disease)(3). However, the mechanisms by which a diet with high saturated fat and cholesterol affects the brain are still poorly understood.
Among the literature data, information about the effect of diet on the brain is the most contradictory in comparison with other organs. For a long time it has been assumed that metabolism in the brain is glycocentric, insulin is independent, aerobic. Only in recent years, new evidence suggested possibility of metabolism in the brain along the lipocentric pathway, in which glycolysis is noted and lipolysis and B-oxidation of fatty acids are activated (4).
Graphical abstract
Naturally, the question arises about the nature of elementary changes in various structures of the central nervous system and their reversibility. As it is known, any changes in the brain occur around the micro-axis of the vessel-astrocytes-neuron (5, 6). It is the interaction of the elements of this micro-axis that ensures the formation of adaptive (adaptive) and compensatory (substitution) reactions in the central nervous system. In this case, the leading role is assigned to the blood vessel at the level of microhemocirculation, where the continuous unity of blood supply, metabolism and neurohumoral regulation is the most clearly manifested (7, 8).
How a diet with a high fat content affects the morphological features of the microvascular network of the brain is still being studied, and the effect of a diet with a 100% fat content has not been previously conducted.
Our study was aimed to investigate the effect of an isolated fat diet on lactate blood level and microcirculation of the rats brain describing in the form of histomorphological changes.
Methods
Study design and population
Interventional prospective (examinations on the 30th day of the experiment), single-sample controlled randomized trial.
Randomization of animals was carried out by the method of random numbers. The numbers of the cells with rats and free cells were entered into the computer, then a random number generator was started, according to which the rats were transferred to the desired cells. 2 groups of animals were formed.
Studied populations
One rat population was studied, divided into 2 groups — 1 experimental and 1 control.
Inclusion criteria: lineal Wistar white rats, male sex, age 7-8 months, weighing 200-250 gr.
The method of forming a sample from the studied population
Arbitrary
1. The control group (n=10) were intact healthy rats, in which the values of the studied parameters were used to calculate control values.
2. The experimental group (n=9) were rats who were assigned a diet consisting exclusively of animal fat (Fig. 1).
Place and time of the experiments carried out
The venue of the study was Kyrgyz State Medical Academy named after IK Akhunbayev, Bishkek, Kyrgyzstan.
The time of the study: September 5, 2023 — October 5, 2023.
Ethics
The work was performed on white, linear Wistar sexually mature 7-month-old male rats weighing 200-250 gr in compliance with the following documents: 1) International recommendations on conducting biomedical research using animals. Chronicle of WHO -1985-Vol.39,-NS-p.3-9.
2) Directive of the European Parliament of 09/22/2010 No. 2010/63 EN "On the protection of animals used for scientific purposes".
The study was approved by the local Ethics Committee of the Kyrgyz State Medical Academy named after IK Akhunbayev (protocol of the meeting No. 52 dated 05/27/2023).
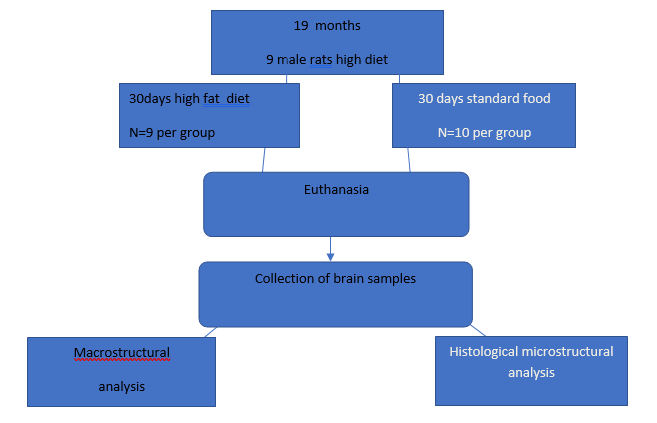
Figure 1. The order of experimental procedures according to the ARRIVE protocol
Description of diet intervention
After one week of acclimatization in a room with an air temperature of 25-30 °with a 12-hour light regime(day/night), the animals were randomly divided in experimental (n=9) and control (n=10) groups (Table 1).
As indicated in Table 1, the animals of the main group received an exclusively fatty diet (fat-mutton fat) for 30 days with free access to water. The data obtained from animals kept on standard feed were used as a control.
|
Table 1. Characteristics of the study groups |
||||
|
Study group |
Type of feed |
Kcal/1 g |
Number of rats |
Observation period |
|
Experimental group |
Protein 0%, carbohydrates 0%, fat 100% |
8,9 Kcal |
9 |
30 days |
|
Control group |
protein 26%, carbohydrates - 64%, fat 10% |
3,06 Kcal |
10 |
30 days |
|
|
Note: the standard feed consisted of bread made from wheat flour of the second grade, oatmeal, cow's milk, salt, herbs, meat – young pork. |
|||
Food intake and body weight were measured daily during the study period. Blood was collected from the cervical vein in glass tubes with lids.
After the 30 days of diet, all animals (n=19) were taken from the experiment. They were sacrificed under general anesthesia (chloroform), and blood was previously taken to determine the lactate level in the AQUA lab.
Body weight and lactate level
Blood was taken to determine the lactate level in the AQUA lab. Blood serum was obtained from rat blood by centrifugation at 3000 rpm for 15 minutes.
To determine whether short-term consumption of an exclusively fat diet could increase body weight, rats from two groups were weighed on the day of the start of the diet (day 0) and at the end of the experiment (on day 30).
Histological analysis
Then, in 6 animals (3- from experimental group and 3- from control group), the bloodstream was injected with a suspension of black ink on 10% neutral formalin in a ratio of 1:4 through the abdominal aorta. Pieces of the brain were fixed in a 10% neutral formalin solution, dehydrated in alcohols of increasing concentration, followed by the preparing paraffin blocks. Histological sections with a thickness of 5-7 mm were stained with hematoxylin-eosin and studied under a light microscope (Olympus, Tokyo, Japan). At
the same time, photography was carried out using a Levenhuk C130 NG digital camera coupled with an optical microscope system (40x magnification) and a computer.
We evaluated changes of microvasculature, endothelium, vessels narrowing or dilation, blood clots and histological changes.
Statistical analysis
Statistical processing of the data was carried out according to the SPSS 22 software (IBM, New York, USA). Due to the fact that the obtained data obeyed the law of normal distribution (according to the Shapiro-Wilk test), a parametric t-criterion for independent samples was used to calculate the reliability of the differences. Continuous variables are denoted by the mean and standard error of mean or median (upper and lower quartiles) and percentage. An independent two-sample test was used for comparing amount of food intake and total body weight gain between the main and control group. The differences in the results were considered significantly significant at p<0.05.
Results
The effect of diet on weight and lactate levels.
The rats of the control and dietary groups were matched for the same body weight at the beginning of the experiment.
The animals of the experimental and control groups were weighed before and after the experiment and the increase in total body weight was determined. After 4 weeks, the rats fed with standard food gained better, and the rats fed exclusively with animal fat did not gain the body weight (increase in total body weight when comparing the diet on day 0 and the diet on day 30; standard diet: 12.93 (0.84)%, exclusively fat diet: 5.98 (0.76)%, (p<0.001) (Table 2).
|
Table 2. The effect of an isolated fat diet on body weight gain in animals of the experimental and control groups |
|||
|
Indicator |
Study groups |
p |
|
|
Experimental |
Control |
||
|
Food intake, g/day |
9.50 (0.54) |
12.54 (0.4) |
< 0.001
|
|
Total body weight gain, % |
5.98 (0.76) |
12.93 (0.84) |
|
|
Data are expressed as the mean (SEM) and n (%) |
|||
The lactate level is known to be used as a marker of the metabolic state of cells, so it was measured by us in animals of both study groups. In animals of the
isolated fat diet, lactate levels were 2.1 times higher than the level of lactate in basal plasma in control animals (Table 3).
|
Table 3. The effect of an exclusively fatty diet on blood lactate in animals of the experimental and control groups
|
||
|
Study groups |
Serum lactate level, mmol/l |
p |
|
Experimental group Isolated fat diet, (n=9) |
1.8 (1.30-2.30) |
<0.001 |
|
Control group Standard diet, (n=10) |
0.84 (0.79 – 0.91) |
|
|
Data are expressed as the median (upper and lower quartiles) |
||
Undesirable phenomena
Of the 20 rats, one rat died on the 15th day of the experiment.
At the end of the experiment, the surviving animals had a tousled coat of slightly yellowish light. Baldness was noted on the abdomen and tail. Upon autopsy, there was a decrease in subcutaneous fat and fatty suspension of the colon and the fatty layer of the mesentery of the small intestine, which looked empty, swollen and transparent.
The effect of a fat diet on the histological characteristics of microvessels
First of all, when studying histological preparations, there is an extreme heterogeneity in the remodeling of the bloodstream of different parts of the brain. There are a number of differences in intravascular, vascular, and extravascular changes depending on the localization of blood vessels. The changes were most pronounced in the vessels of the sensory zone, in the vessels of the plexuses of the ventricles and the soft meninges, as well as the diencephalic region (Fig. 2).
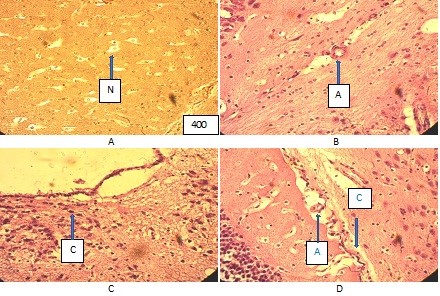
Figure (microphoto) 2. Cytoarchitectonics of the brain of the vascular plexus of the 4th ventricle and the microcirculatory system of the control group rat. Hematoxylin eosin. Filling in paraffin. X400 (A – parenchyma of the brain with neurons (N), Van Gieson staining, B – arteriole (A), C – epithelial cells of the 3rd ventricle, D –blood vessels (A – artery, C - capillary)
Histologically, microvessels in rats showed varying degrees of pathology of endothelial cells of microcirculatory vessels, including their edema, epithelial peeling and vacuolization of walls, as well as changes in vascular tone with their narrowing of some vessels and dilation of others (Fig. 3, 4, 5).

Figure (microphoto) 3. Arteriole (A) in the sensory area of the cerebral cortex is spasmodic, the lumen of the arteriole is closed. Endothelial cells are edematous. Van Gieson staining. Filling with paraffin. X400
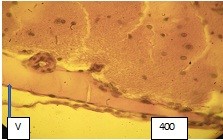
Figure 4 (microphoto). Vein wall (V) with pronounced edema, uneven arrangement of the endothelium, vacuoles in the vessel wall are determined. There is swelling and exfoliation of the endothelium around the vessel into the lumen of the vessel. Van Gieson staining. Filling with paraffin. X400
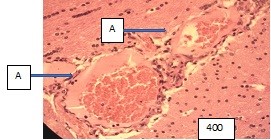
Figure (microphoto) 5. Hypothalamic region, vessels (A – artery) are characterized by extreme dilation with a stretched wall and separation of blood into the lumen. Hematoxylin eosin staining. Filling in paraffin. X400
Additional changes were due to endothelial dysfunction, the local procoagulation state of the blood with a slowdown in blood flow in them, the formation of aggregates from blood cells elements, which eventually lead to impaired perfusion of the surrounding brain tissue and the development of its edema and dystrophy (Fig. 6, 7, 8, 9).
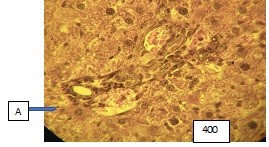
Figure (microphoto) 6. In the arteries (A) endothelial cells in the form of a palisade, there is a folding of the inner membrane. There is a sludge phenomenon of erythrocytes in the lumen of the arteries, plasma (blood separation) along the periphery of the vessels; surrounding brain tissue with dystrophic changes. Van Gieson staining. Filling in paraffin. X400
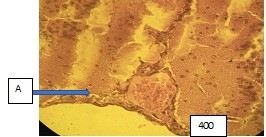
Figure (microphoto) 7. Vessel (A) with signs of blood separation, dystrophy and foci of ischemia in the brain tissue. Van Gieson staining. Filling in paraffin. X400
We also observed that degenerative changes in the endothelium were accompanied by thickening of the basement membrane, and in some vessels by its ruptures (Fig. 8, 9).
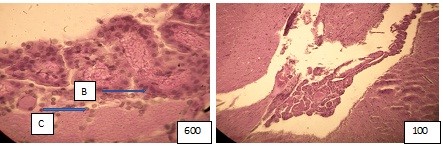
Figure (microphoto) 8. The capillary wall (C) of the vascular plexus 4th ventricle with a thickened basement membrane (B) (left), light cytoplasm, swollen nuclei, erythrocyte sludge in the lumen (P) (right), plasma in some of them. Hematoxylin eosin. Filling in paraffin. X600 and 100
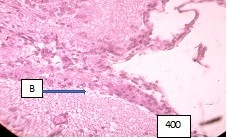
Figure 9 (microphoto). In the vessels of the soft meninges, the basement membrane is of different thickness, part of the lumen of the vessels is neglected, there are areas of wall ruptures, the underlying part of the brain is edematous. Hematoxylin eosin. Filling in paraffin. X400
Pathology of endothelial cells with thickening of the basement membrane and expansion of the perivascular spaces of the brain vessels were characteristic signs in animals that were on an isolated fatty diet (Fig. 10)
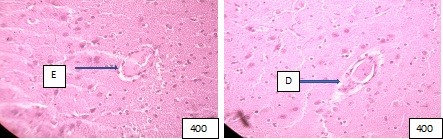
Figure (microphoto) 10. Edema (E) and exfoliation of the endothelium into the lumen of the vessel (left), (D) wall dissection with abnormality of the integrity of the endothelium are observed around the vessel (right). Hematoxylin eosin. Filling in paraffin. X400
Discussion
In comparison with the control group, contrary to what was expected in the rats of the dietary intervention group, there was not an increase, but a decrease in total body weight gain after 30 days of diet. The lower body weight in rats fed exclusively animal fat observed in this study can be explained by a lower intake of protein, which is necessary to maintain muscle growth, especially in young organisms.
We also measured the serum lactate concentration in animals of both study groups. Lactate is the main circulating substrate and energy exporter after glucose, and acts as a marker and modulator of oxygen availability and its utilization (9). In the animals of the experimental group, an increase in lactate levels was noted, which is a consequence of metabolic changes against the background of an unbalanced diet in favor of fats and the absence of other important macronutrients such as glucose and protein.
Astrocytes and neurons are known to use lactate significantly more than other metabolites such as glucose, 3-hydroxybutyrate or glutamine. Therefore, in conditions of protein and glucose deficiency, lactate is constantly supplied from the blood to both neurons and astrocytes for use during brain activity (10).
An isolated fat diet observed for 30 days led to a change in the morphology of cerebral microvessels and arteries in experimental animals (Wistar rats) and to a number of pathological transformations. This diet contributed to the remodeling of the microvascular bed, which manifested itself in a violation of the integrity of the endothelium and, as a result, may increase the incidence of related cerebrovascular diseases. As indicated by the literature data, consumption of high amount of animal fat can raise cholesterol, clog arteries and contribute to the risk of stroke and other forms of heart disease – not to mention obesity, diabetes, cancer and a host of other conditions (10). A higher intake of vegetable fat, polyunsaturated fat, and vegetable oil was associated with a reduced risk of stroke, but a high intake of non-dairy animal fat, total red meat, and processed red meat was associated with increased stroke risk (11).
Our results show that a high-fat diet contributes to the development of pathological rearrangements in the vessels of the microcirculatory bed, where more serious changes occur than in large and medium-sized arteries. In the group of animals with an isolated fat diet, the endothelium of the microvessels of the brain was edematous with a large number of vacuoles, there were edema of cell nuclei, and a thickened vascular basement membrane, as well as an expanded perivascular space and edematous brain tissue with signs of its dystrophy. As is known, intima plays a key role not only in its selective permeability for communication between blood and the vessel wall, but also in the secretion of effector molecules, as well as the regulation of blood vessel tone and blood flow. Its participation in the processes of inflammation, immunity, thrombosis and angiogenesis is known (12-15). Any pathology of the endothelium can increase the likelihood of slowing blood flow and platelet aggregation, which eventually leads to the formation of a blood clot. Since the animals were subjected to a diet for a relatively short period of time, nevertheless, signs of dystrophic changes in the substance of the brain were noticed, which indirectly may indicate the development of hyperlipidemia, which led to the remodeling of microvessels.
Comparison with other publications
Studies devoted to the effect of an extreme (100%) fat diet on the morphological characteristics of the microcirculatory bed have not been conducted before, and our study extends and contributes to the existing knowledge on change in brain vessels. Few studies have been conducted on a high-fat diet for brain vessels, the available ones have shown that such a diet can in turn lead to impaired microcirculation, including endothelial dysfunction, impaired vascular tone and reactivity, increased blood flow, decreased functional capillary density and dilution of microvascular density, a significant increase in permeability to macromolecules, as well as vasoconstriction (14-16). Hyperlipidemia is best known for its role in the development of atherosclerotic vascular diseases, a process that is most often observed in large arteries such as the aorta or coronary arteries (13-16). However, the processes associated with atherosclerosis (arteriosclerosis affecting large and middle sized arteries) may differ slightly from the processes occurring in arteriolosclerosis (arteriosclerosis affecting small arteriolar branches), and the relationship between dyslipidemia and arteriolosclerosis is less obvious. There is some evidence that hypercholesterolemia can also cause both functional and structural changes in the peripheral microcirculatory bed (17). It is generally believed that cholesterol does not cause pathology of the microcirculatory bed.
Study limitations
Limitations may be related to the probability of inconsistency of theoretical reflections with practical conclusions. Since it was not possible to keep 1 rat in 1 a cage, there could be restrictions on the consumption of animal feed during the day.
Limitations in the conditions of the experiment
In addition, the individual characteristics of rats in terms of the amount of nutrition and metabolic processes introduced some errors into the study. The conditions of animal decapitation, blood collection, and the quality of serum production for the study could be the reason for the bias in the results. However, these biases of the restriction could affect the results only from the point of view of their statistical significance, the dynamics of changes would remain the same.
Directions for further research
In the future, it is planned to study the metabolism and histomorphology of the brain in experimental animals fed by isolated carbohydrate and protein diets.
Conclusions
1. The lower body weight in rats fed exclusively on a fat diet can be explained by a lower intake of protein, which is necessary to maintain muscle growth, especially in young organisms
2. In the animals of the experimental group, an increase in lactate levels was noted, which is a consequence of metabolic changes against the background of an unbalanced diet in favor of fats and a deficiency of other important macronutrients such as glucose and protein.
3. High fat intake can cause functional and structural changes in the vascular network.
4. It can lead to a number of pathological changes and destruction of microvessels, which can increase the likelihood of slowing down blood flow and platelet aggregation and, ultimately, to the development of thrombosis.
Ethics: The experimental work was performed in compliance with the international rules on conducting animal research: 1) International recommendations on conducting biomedical research using animals. Chronicle of WHO -1985-Vol.39,-NS-p.3-9.
2) Directive of the European Parliament of 09/22/2010 No. 2010/63 EN "On the protection of animals used for scientific purposes".
The study was approved by the local Ethics Committee of the Kyrgyz State Medical Academy named after IK Akhunbayev (protocol of the meeting No. 52 dated 05/27/2023).
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: equally contributed to manuscript preparation and fulfilled the authorship criteria. All authors approved the final version of the article before publication, and agreed to be responsible for all aspects of the work, implying proper study and resolution of issues related to the accuracy or integrity of any part of the work.
Acknowledgement: None to declare
Funding: The work was carried out within the framework of a project funded by the Ministry of Science and Education of the Kyrgyz Republic on the topic: "Alimentary dystrophic syndromes and their reversibility: disruption and restoration of interconnection at different levels of the organization after the action of diet as a key factor of exposome."
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Not applied
References
| 1.Wali JA, Jarzebska N, Raubenheimer D, Simpson, S.J, Rodionov RN, O'Sullivan JF. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-a narrative review. Nutrients 2020; 12: 1505. doi:10.3390/nu12051505 https://doi.org/10.3390/nu12051505 PMid:32455838 PMCid:PMC7284903 |
||||
| 2.Huttasch M, Roden M, Kahl S.Obesity and MASLD: Is weight loss the (only) key to treat metabolic liver disease Metabolism 2024; 157: 155937. doi:10.1016/j.metabol.2024.155937 https://doi.org/10.1016/j.metabol.2024.155937 PMid:38782182 |
||||
| 3.Zeng XF, Varady KA, Wang XD, Targher G, Byrne CD, Tayeem R, et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: An international multidisciplinary expert consensus. Metabolism 2024; 161: 156028. https://doi.org/10.1016/j.metabol.2024.156028 PMid:39270816 |
||||
| 4.Speakman JR. Use of high-fat diets to study rodent obesity as a model of human obesity. Int J Obes (Lond) 2019; 43: 1491-92. https://doi.org/10.1038/s41366-019-0363-7 PMid:30967607 |
||||
| 5.Fifield KE, Rowe TM, Raman-Nair JB, Hirasawa M, Vanderluit JL. Prolonged high fat diet worsens the cellular response to a small, covert-like ischemic stroke. Neuroscience 2019; 406: 637-52. doi: 10.1016/j.neuroscience.2019.01.050 https://doi.org/10.1016/j.neuroscience.2019.01.050 PMid:30731155 |
||||
| 6.De Aquino CC, Leitão RA, Alves LAO, Coelho-Santos V, Guerrant RL, Ribeiro CF, et al. Effect of hypoproteic and high-fat diets on hippocampal blood-brain barrier permeability and oxidative stress. Front Nutr 2019; 5: 131. https://doi.org/10.3389/fnut.2018.00131 PMid:30687711 PMCid:PMC6333637 |
||||
| 7.Birulina J.G., Ivanov V.V., Buyko E.E., Bykov V.V., Dzyuman A.N., Nosarev A.V., Grigoreva A.V., Gusakova S.V. Morphological changes in the heart and aorta of rats with diet-induced metabolic syndrome. Bull Siberian Med 2022; 21:13-21. doi:10.20538/1682-0363-2022-3-13-21 https://doi.org/10.20538/1682-0363-2022-3-13-21 |
||||
| 8.Zimmerman B, Kundu P, Rooney WD, Raber J. The effect of high fat diet on cerebrovascular health and pathology: a species comparative review. Molecules 2021; 26: 3406. https://doi.org/10.3390/molecules26113406 PMid:34199898 PMCid:PMC8200075 |
||||
| 9.Wu P, Zhu T, Huang Y, Fang Z, Luo F. Current understanding of the contribution of lactate to the cardiovascular system and its therapeutic relevance. Front Endocrinol (Lausanne) 2023; 14: 1205442. doi: 10.3389/fendo.2023.1205442 https://doi.org/10.3389/fendo.2023.1205442 PMid:37396168 PMCid:PMC10309561 |
||||
| 10. Yamagata K. Lactate supply from astrocytes to neurons and its role in ischemic stroke-induced neurodegeneration. Neuroscience 2022; 481: 219-31. doi:10.1016/j.neuroscience.2021.11.035. https://doi.org/10.1016/j.neuroscience.2021.11.035 PMid:34843897 |
||||
| 11.Zhou-Qing Kang, Ying Yang, Bo Xiao, Dietary saturated fat intake and risk of stroke: Systematic review and dose-response meta-analysis of prospective cohort studies, Nutrition Metab Cardiovasc Dis 2020; 30: 179-89. doi:10.1016/j.numecd.2019.09.028 https://doi.org/10.1016/j.numecd.2019.09.028 PMid:31791641 |
||||
| 12.Grisotto C, Taïlé J, Planesse C, Diotel N, Gonthier MP, Meilhac O, et al. High-fat diet aggravates cerebral infarct, hemorrhagic transformation and neuroinflammation in a mouse stroke model. Int J Mol Sci 2021; 22: 4571. doi: 10.3390/ijms22094571 https://doi.org/10.3390/ijms22094571 PMid:33925459 PMCid:PMC8123851 |
||||
| 13.Fewkes JJ, Kellow NJ, Cowan SF, Williamson G, Dordevic AL. A single, high-fat meal adversely affects postprandial endothelial function: a systematic review and meta-analysis. Am J Clin Nutr 2022; 116:699-729. doi: 10.1093/ajcn/nqac153. https://doi.org/10.1093/ajcn/nqac153 PMid:35665799 PMCid:PMC9437993 |
||||
| 14.De Montgolfier O, Pinçon A, Pouliot P, Gillis M-A, Bishop J, Sled JG., et al. High Systolic blood pressure induces cerebral microvascular endothelial dysfunction, neurovascular unit damage, and cognitive decline in mice. Hypertension 2019; 73:217-28. https://doi.org/10.1161/HYPERTENSIONAHA.118.12048 PMid:30571552 |
||||
| 15.Tain YL, Hsu CN. Maternal high-fat diet and offspring hypertension. Int J Mol Sci 2022; 23:8179. doi: 10.3390/ijms23158179. https://doi.org/10.3390/ijms23158179 PMid:35897755 PMCid:PMC9332200 |
||||
| 16.Sighinolfi G, Clark S, Blanc, L, Cota D, Rhourri-Frih B. . Mass spectrometry imaging of mice brain lipid profile changes over time under high fat diet. Sci Rep 2021; 11: 19664 doi:10.1038/s41598-021-97201-x https://doi.org/10.1038/s41598-021-97201-x PMid:34608169 PMCid:PMC8490458 |
||||
| 17.Jebari-Benslaiman S, Galicia-García U, Larrea-Sebal A, Olaetxea JR, Alloza I, Vandenbroeck K, et al. Pathophysiology of atherosclerosis. Int J Mol Sci 2022; 23: 3346. doi: 10.3390/ijms23063346 https://doi.org/10.3390/ijms23063346 PMid:35328769 PMCid:PMC8954705 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

