The influence of sports-related factors on left heart chambers in young female athletes
ORIGINAL RESEARCH ARTICLE
The influence of sports-related factors on left heart chambers in young female athletes
Article Summary
- DOI: 10.24969/hvt.2024.528
- CARDIOVASCULAR DISEASES
- Published: 08/12/2024
- Received: 15/10/2024
- Revised: 30/11/2024
- Accepted: 02/12/2024
- Views: 3325
- Downloads: 2235
- Keywords: Athletic heart syndrome, female athlete, cardiac remodeling, training type, endurance training, mixed training, total training duration, weekly training load
Address for Correspondence: Damirbek Abibillaev, Ala-Too International University, Bishkek, Kyrgyzstan
Email: damirbek.abibillaev@alatoo.edu.kg Mobile: +996700579004
ORCID: Damirbek Abibillaev - 0000-0002-4660-3064, Aida Baatyrbekova - 0000-0002-1694-567X, Aisanam Abdurasulova - 0000-0002-4728-4026, Kudaibergen Osmonaliev - 0009-0008-4469-1065, Ryskul Kydyralieva - 0000-0003-4959-1449, Taalaibek Kudaiberdiev - 0000-0002-3669-066X
Damirbek Abibillaev1a,2a,3, Aida Baatyrbekova2b , Fuat Kocyigit4, Sergei Petrovsky5, Andrey Polubabkin6, Aisanam Abdurasulova1a, Kudaibergen Osmonaliev1b, Ryskul Kydyralieva1a, Taalaibek Kudaiberdiev7
1aDepartment of Therapeutic Disciplines and Family Medicine and 1bDepartment of Surgical Disciplines and Obstetrics&Gynecology, Faculty of Medicine, Ala-Too International University, Bishkek, Kyrgyzstan
2aMedical Center and 2bDepartment of Facultative Therapy, I.K.Akhunbaev Kyrgyz State Medical Academy, Bishkek, Kyrgyzstan
3Consultative and Diagnostic Department, Research Institute of Heart Surgery and Organ Transplantation, Bishkek, Kyrgyzstan
4Consulting Research and Statistics Center, International School of Medicine, International University of Kyrgyzstan, Bishkek, Kyrgyzstan,
5B.T.Turusbekov Kyrgyz State Academy of Physical Culture and Sports, Bishkek, Kyrgyzstan
6Athletic Federation of Kyrgyz Republic, Bishkek, Kyrgyzstan
7Heart, Vessels and Transplantation, Center for Scientific Research and Development of Education, Bishkek, Kyrgyzstan
Abstract
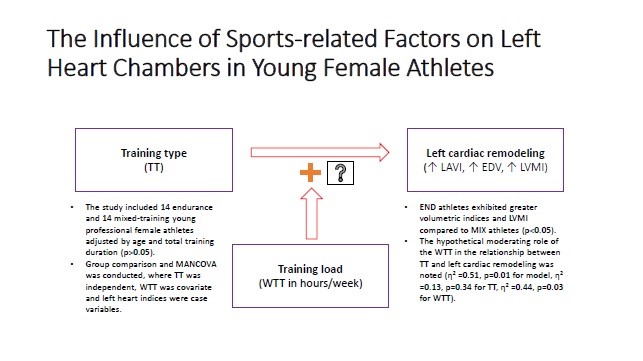
Objective: Athletic heart syndrome (AHS) represents a physiological and morphological adaptation of the heart to long-standing intensive training. Left ventricular (LV) chamber sizes are primarily affected by the complex interplay of sports-associated factors. AHS in females substantially varies from males due to underlying physiological, hormonal, and anthropometric differences. This study aims to explore the influence of sports-related factors on left heart chambers in young female athletes (FA).
Methods: A cross-sectional study was conducted in 28 young FA, who undergo training for at least 3 months with a minimum of 3 times weekly sessions. They were divided in 2 groups: endurance (END) and mixed-training (MIX). We assessed using echocardiography: left atrial volume index (LAVI), end-diastolic (EDV) and end-systolic (ESV) LV volumes, LV mass index (LVMI). Comparisons were performed using t-tests or Mann-Whitney U tests. A MANCOVA was selected to determine the relationship between sports-related factors and left heart indices. Post-hoc contrast and margins tests were conducted to show a correlation with exact cardiac parameters.
Results: Groups were matched by age, body surface area, and total training duration. However, weekly training time (WTT) was higher in END athletes (12.7 (5.3) vs. 5.7 (0.8), p<0.001) and left cardiac indices were greater in END athletes than in MIX counterparts: LAVI (28.8 vs.18.7 ml/m2, p=0.009), EDV (81.1 (11.7) vs 66.1 (16.8) ml, p=0.01), ESV (29.6 (10.8) vs. 20.5 (10) ml, p=0.01) and LVMI (68.3 (11.7) vs. 59.2 (8.2) gr/m2, p=0.02). According to MANCOVA, higher WTT (p=0.03, η² = 0.44), was associated with increased LAVI, EDV, and LVMI values. Mean differences obtained from contrast analysis showed significant variations of only LVMI between training groups (-13.17, p=0.04). Post-hoc analysis revealed significantly higher values of all chamber parameters in END athletes (p<0.001).
Conclusions: Young FA have distinct cardiac adaptations based on training type and training load time. END athletes exhibited greater volumetric indices and LVM compared to MIX athletes, emphasizing the significant influence of training intensity and modality on cardiac remodeling. Furthermore, the hypothetical moderating role of the WTT in the relationship between training type and left heart chamber sizes was observed. These findings emphasize the importance of individualized cardiac evaluation in FA for the adequate management of the AHS.
Key words: Athletic heart syndrome, female athlete, cardiac remodeling, training type, endurance training, mixed training, total training duration, weekly training load
Graphical abstract
Introduction
Undoubtedly, long-standing physical training induces physiological adaptations in various organ systems, including the cardiovascular system. Among athletes, the heart undergoes specific structural and functional changes, often referred to as “athletic heart syndrome” (AHS) (1, 2). These adaptations are largely dependent on the type and intensity of training, with various training types (TT) eliciting different cardiac alterations. As accepted conventionally, endurance sports, such as field and track athletics or long-distance swimming, induce volume overload on the heart, leading to eccentric hypertrophy (3, 4).
Although endurance and power training are well-documented to cause specific cardiac changes such as eccentric and concentric morphological alterations, the effects of mixed training, which combines both endurance and strength elements, remain less understood. Most of the studies were conducted in a comparative mode in Caucasian and Black ethnicities (5, 6). It is unclear whether athletes engaging in mixed sports develop cardiac adaptations more similar to endurance athletes or those engaging in strength training.
Studies have demonstrated that at least three or four months of vigorous training is sufficient for the development of AHS (2). Differences in hormonal levels, body composition, and training responses can cause female athletes to exhibit different cardiac adaptations compared to their male counterparts. Studies have shown that female athletes generally develop less pronounced concentric cardiac remodeling and hypertrophy than males, despite engaging in similar training intensities (7, 8). However, little is known about the AHS in young female athletes.
This study aims to elucidate the functional and structural cardiac remodeling through transthoracic echocardiography (TTE) in young female athletes of endurance (END) and mixed (MIX) training. We hypothesize that END athletes will display greater changes in left ventricular dimensions than MIX counterparts. Furthermore, training load along with TT might exert a significant impact on cardiac remodeling in professional athletes.
Methods
Study population and design
Twenty-nine female athletes from the Republican Sports College of the Olympic Reserve were enrolled in this cross-sectional study conducted during September and October of 2022.
Athletes of track and field, swimming, and football disciplines were selected through a convenient sampling method. Athletics (field and track sports) and swimming disciplines were considered as END training, whereas football was accepted as a form of MIX training. This matching of sports disciplines with training types was based on European Society of Cardiology Guidelines criteria (9). Inclusion criteria for the study were female athletes of either endurance or mixed training, aged between 14 and 20 years, at least 3 months of regular exercise with a minimum of 3 training sessions or 6 hours per week (2), and lack of prior history of cardiovascular disease or other chronic medical conditions.
The study was approved by the Institutional Review Board of the Ala-Too International University. All participants provided informed consent before participating in the study. Data was collected anonymously and stored securely to ensure confidentiality.
Anthropometric, hemodynamic and sports-related indices
All subjects were evaluated for basic anthropometric indices, such as age, body weight, height and body surface area (BSA) (according to Mosteller formula), pre-procedure systolic (SBP) and diastolic blood pressures (DBP) in mmHg, heart rate in beats per minute (bpm). Sports-related indices included overall training time duration (TTD) expressed in months, weekly training time (WTT) a surrogate marker of training load expressed in hours, and number of participated competitions (CP) regardless of the level (local, republic, international).
Echocardiographic Assessment
All athletes underwent a comprehensive TTE evaluation using a standardized protocol recommended by EACVI/ASE guidelines (9). Echocardiograms were performed by a single experienced cardiologist from the Research Institute of Heart Surgery and Organ Transplantation to ensure consistency in measurements. Philips CX50 with both adult and pediatric phased-array probes were used for the investigation procedure.
The following morphological echocardiographic parameters of the left heart were evaluated: left ventricular end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), end-diastolic volume (LVEDV), end-systolic volume (LVESV), ejection fraction (LVEF), interventricular septum thickness (IVS), left ventricular posterior wall thickness (LVPW), left ventricular mass index (LVMI), relative wall thickness (LVRWT), left atrial anteroposterior diameter (LA) and volume index (LAVI), and aortic diameters at four levels as recommended by guidelines: aortic fibrous annulus (AFA), sinuses of Valsalva (SV), sino-tubular unction (STJ) and proximal ascending aorta (PAA) (10,11).
Furthermore, left ventricular global longitudinal strain (GLS) was evaluated offline by an experienced operator. The QLAB pack of speckle-tracking echocardiographic modality based on a 17-segment model was applied for offline strain analysis. Evaluation and interpretation of reports guided under current guidelines (12).
Statistical analysis
All tests were performed using Stata 16.1 software (StataCorp, Texas, USA). Categorical variables are highlighted via absolute count (%) and continuous variables are reported as mean (standard deviation) in case of normal distribution whereas median (interquartile range) in case of the violation of normality assumption. Comparison of variables between groups was achieved by independent samples t-test or Mann-Whitney U test, depending on the results of the Shapiro-Wilk test for normality of distribution. To investigate the potential correlations between TT, confounders, and cardiac chamber indices we conducted multiple analysis of covariance (MANCOVA) test. After the MANCOVA test, post-hoc contrast and margin analyses were performed. Partial eta square (η²) was calculated for the estimation of effect size after MANCOVA. The statistical significance was adjusted for the p-value of less than 0.05 and a confidence interval of 95%.
Results
Demographic, anthropometric, hemodynamic and sports-related findings
Due to the accidental finding of a congenital bicuspid aortic valve with significant ascending aortic dilatation during TTE evaluation, one endurance athlete was excluded from the study. Overall distribution according to sports disciplines is presented as follows: eight track and field athletes, six swimmers, and fourteen football players. Thus, both training groups equalized by absolute count (n=14 for each END and MIX group).
Groups were comparable by age and anthropometric parameters (Table 1). The mean age of athletes was 16.5 (2.3) years without significant differences by groups (p=0.25). None of the weight, height, BSA showed statistical differences by groups (p=0.78, p=0.88, p=0.97, respectively).
|
Table 1. Comparison analysis among by training groups |
||||
|
Parameter |
Total (n=28) |
END (n=14) |
MIX (n=14) |
p |
|
Age, years |
16.5(2.3) |
17(2.7) |
16(1.7) |
0.25 |
|
Weight, kg |
52.9(6.9) |
52.5(3.8) |
53.3(9.1 |
0.78 |
|
Height, cm |
164.9(5.05) |
165.07(4.9) |
164.7(5.3 |
0.88 |
|
BSA, m2 |
1.55(0.11) |
1.55(0.06) |
1.55(0.15) |
0.97 |
|
SBP, mm Hg |
103.4(7.5) |
101.9(8.4) |
105(6.5) |
0.29 |
|
DBP mm Hg |
62.3(7.1) |
60(8.7) |
64.6(4.1) |
0.08 |
|
HR, bpm |
70.3(11.1) |
65.7(12.6) |
74.9(7.2) |
0.02 |
|
TTD, months |
48 (16-60) |
48 (12-54) |
36 (24-60) |
0.67 |
|
WTT, hours |
9.2(5.2) |
12.7(5.3 |
5.7(0.8 |
≤0.001 |
|
CP |
3.5 (1.5-10.5) |
9.5 (0-11) |
2 (2-5) |
0.12 |
|
AFA, mm |
17.6 (16.8-18.2) |
18.2 (17.1-19.5) |
17.1 (16.8-17.8) |
0.01 |
|
SV, mm |
26.9(1.7) |
27.6(2.02) |
26.2(2.2) |
0.08 |
|
STJ, mm |
23.5(1.7) |
24.1(1.5) |
22.9(1.7) |
0.07 |
|
PAA, mm |
23.8 (22.4-24.7) |
24 (23.3-24.9) |
23.1 (21.9-24.6) |
0.34 |
|
LA, mm |
29.7(2.9) |
30.1(3.2) |
29.3(2.8) |
0.46 |
|
LAVI, ml/m2 |
21.2 (17.7-28.8) |
28.8 (21.6-32.4) |
18.7 (17.6-20.9) |
0.009 |
|
LVEDD, mm |
45.3(3.07) |
45.7(3.6) |
44.9(2.4) |
0.48 |
|
LVESD, mm |
27 (25.4-29.3) |
26.2 (25.4-28.2) |
28.1 (25.5-29.4) |
0.42 |
|
IVS, mm |
7.4(0.93) |
7.9(0.88) |
7.03(0.77) |
0.009 |
|
LVPW, mm |
7.5(0.87) |
7.8(0.79) |
7.2(0.84) |
0.03 |
|
LVMI, gr/ m2 |
63.8(10.9 |
68.3(11.7) |
59.2(8.2) |
0.02 |
|
RWT |
0.32(0.04) |
0.33(0.04) |
0.31(0.03) |
0.13 |
|
EDV, ml |
73.6(16.2) |
81.1(11.7) |
66.1(16.8) |
0.01 |
|
ESV, ml |
24.7 (20.05-30.45) |
29.6 (22.1-32.9) |
20.5 (18.7-28.7) |
0.01 |
|
EF, % |
65.4(3.5) |
64.8(3.6) |
65.9(3.4) |
0.41 |
|
GLS, % |
20.9(2.8) |
20.9(2.7) |
20.9(2.9) |
0.95 |
|
AVA – aortic valve annulus, BSA – body surface area, CP – competition participation, DBP – diastolic blood pressure, EDV – end-diastolic volume, ESV – end-systolic volume, GLS – global longitudinal strain, HR – heart rate, IVS – interventricular septum, PAA – proximal ascending aorta, LA – left atrium, LAVI – left atrial volume index, LVEDD – left ventricular end-diastolic diameter, LVESD – left ventricular end-systolic diameter, LVMI – left ventricular mass index, LVPW – left ventricular posterior wall, RWT – relative wall thickness, SBP – systolic blood pressure, SV – sinus Valsalva size, STJ – sino-tubular junction, TTD – total training duration, WTT – weekly training time |
||||
Average SBP and DBP values were observed to be slightly higher in MIX than END group without statistical significance (p=0.29, p=0.08, respectively). Resting heart rate was higher in MIX (p=0.02) than in END group.
Sports-related parameters were found with some extent of differences by groups. Overall median of TTD was 48 (16-60) months without significant differences by groups (48 (12-54) vs 36 (24-60), p=0.67). On the other hand, WTT was noted higher in END athletes than MIX ones (12.7 (5.3) vs 5.7 (0.8)h/wk, p<0.001). The median CP also differed by groups, however, the comparison test did not reveal statistical significance (p=0.11).
Comparison of left heart indices according to TT
TTE measurements of the left cardiac chambers were presented with unequivocal findings. Among aortic diameters, only AFA size was found to be significantly varied between groups (p=0.01). Despite the relatively larger aortic sizes at the SV, STJ, and PAA levels in END, no statistical significance was reached (p>0.05).
LA size was found to be indifferent whereas LAVI revealed a significant difference, i.e. END athletes showed larger values of LAVI (28.8 vs 18.7 ml/m2, p=0.009) as compared to MIX athletes. Similar findings were presented by left ventricular indices, where B-mode sizes were indifferent, and volumetric indices were higher in the END group (81.1(11.7) vs 66.1(16.8) ml, p=0.01 for EDV and 29.6 (22.1-32.9) vs20.5 (18.7-28.7) ml (p=0.01) for ESV, as compared to MIX group.
Both IVS and LVPW diameters had higher values in END group (p=0.009 for IVS; p=0.03 for LVPW). Furthermore, it was confirmed by LVMI values in athletic groups (68.3(11.7) vs 59.2(8.2) gr/m2, p=0.02 respectively for END and MIX). Interestingly, functional indices were presented without statistical variations (p=0.41 for EF; p=0.95 for GLS).
Relationship analysis of sports-related factors and echocardiographic athletic heart syndrome
A MANCOVA model consisted of training type as an independent or grouping variable, WTT as a covariate, and LAVI, EDV, and LVMI as continuous test variables (Table 2).
|
Table 2. Characteristics of variables included into the MANCOVA model |
|||
|
Variable role |
Variable name |
Variable type |
Distribution |
|
Group variable |
Training type |
Categorical |
|
|
Confounder variable |
Training load |
Continuous |
Normal |
|
Test variable |
LAVI |
Continuous |
Normal |
|
Test variable |
LV EDV |
Continuous |
Normal |
|
Test variable |
LVMI |
Continuous |
Normal |
All test variables presented normal distribution without significant outliers. The obtained results are highlighted in Table 3, where the overall model fit was acceptable with appropriate statistical significance (p=0.03, η²=0.51). Despite the non-significant results of training type (p=0.34, η²=0.13), WTT clearly showed a significant effect on left heart chamber indices (p=0.03, η² = 0.44). According to these findings, training type had 13%, WTT had 44%, and cumulatively 51% of the impact on chamber variations in these athletic subjects.
|
Table 3. MANCOVA test results |
||||
|
|
Wilk’s lambda |
F |
p |
Effect size |
|
Overall model fit |
0.09 |
2.16 |
0.01 |
0.51 |
|
Training type |
0.81 |
1.19 |
0.34 |
0.13 |
|
Training load |
0.14 |
1.83 |
0.03 |
0.44 |
Post-estimation contrast analysis revealed a significant difference in LVMI between MIX and END groups while controlled by WTT (-19.16 and p=0.007). However, the mean differences of EDV and LAVI did not reach statistical significance (p=0.44 and p=0.06, respectively). The results of contrast analysis are highlighted in Table 4.
|
Table 4. Post-hoc contrast test results |
||||
|
Test variable |
Mean difference1 |
Standard error |
F |
p |
|
LAVI |
-7.6 |
3.98 |
3.64 |
0.06 |
|
LV EDV |
-7.08 |
9.05 |
0.61 |
0.44 |
|
LVMI |
-13.17 |
6.37 |
4.27 |
0.04 |
|
LAVI – left atrial volume index, LV EDV – left ventricular end-diastolic volume, LVMI – left ventricular mass index 1Mean difference was calculated as mixed-endurance |
||||
Post-hoc margin analysis confirmed the findings of contrast analysis where the predicted mean in LAVI for END athletes was higher than MIX athletes (27.35 vs. 19.7 ml/m2, p<0.001), and equivocal results were obtained for EDV (77.18 vs. 70.09 ml, p<0.001) and LVMI (70.4 vs. 57.23 gr/m2, p<0.001) when controlled by WTT (Table 5).
|
Table 5. Post-hoc linear prediction (margins) of test variables |
||||
|
Test variable |
Margins* |
Standard error |
t-test* |
p |
|
LAVI |
27.35/19.75 |
2.32 |
11.75/8.48 |
<0.001 |
|
LV EDV |
77.18/70.09 |
5.28 |
14.59/13.25 |
<0.001 |
|
LVMI |
70.40/57.23 |
3.72 |
18.90/15.36 |
<0.001 |
|
LAVI – left atrial volume index, LV EDV – left ventricular end-diastolic volume, LVMI – left ventricular mass index; *Values were calculated for END/MIX respectively |
||||
According to these findings, we created a hypothetic independent-covariate-dependent or x-y-z variable interaction diagram (Fig. 1). Given the increase of cumulative effect size of the MANCOVA model when the effect of training type was adjusted for WTT, we hypothesized the moderating effect of WTT in the interaction of training type and cardiac chamber indices.
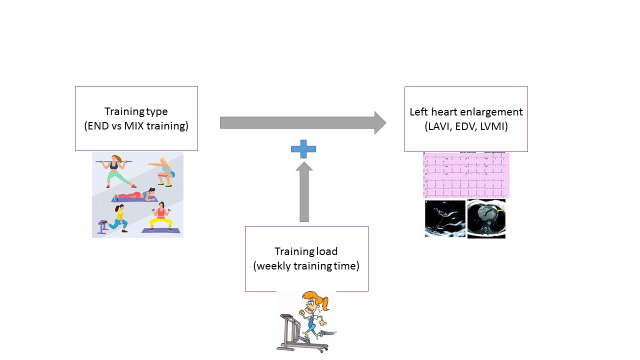
Figure 1. Hypothetical interaction model of sports-related and cardiac parameters
Discussion
Notably, in our study, END athletes were found with both dilation and hypertrophy of left heart chambers, as evidenced by literature (2, 9, 13). AHS typically manifests differently among athletes, with key factors such as training type, sports discipline, athlete’s career, and other several factors influencing these variations (2, 14). Some reports based on the Morganroth hypothesis insisted on the development of eccentric remodeling in the case of endurance and concentric resistance training (3, 4). On the other hand, dynamic and static components of exercise play a crucial role besides aerobic components (14, 15).
Doronina et al. (16) reported that athletes on predominantly static training characterized by concentric remodeling, whereas athletes of high dynamic endurance resulted in eccentric remodeling. Despite the limited information about the training programs of athletes, we assume that cardiac remodeling in END groups resulted from both complex impacts of static-dynamic and aerobic components of field and track sports, as well as swimming.
The observed interaction between TT and WTT on cardiac chamber indices suggests the possible moderating role of WTT. While training type alone explained only 13% of the variability in left heart chamber indices, adjusting for WTT increased the model’s explanatory power to 51%. This highlights the complex interplay between intrinsic (e.g., TT) and extrinsic (e.g., WTT) factors in shaping cardiac adaptations. Such moderating effects have been underexplored in the context of AHS and warrant further investigation (17). Additionally, post-hoc contrast analysis highlighted the significant changes in the LVMI. Post-hoc margin analysis further reinforced these findings, showing significant differences in LAVI, LVMI, and EDV between END and MIX athletes after adjusting for WTT. These results underscore the importance of considering training intensity and duration when evaluating the impact of sports-related factors on cardiac structure.
Strikingly, functional indices such as EF and global GLS did not show significant differences between groups. This suggests that despite structural remodeling, functional adaptations remain preserved, consistent with the physiological nature of AHS in well-trained athletes. The findings from this study provide valuable insights into the cardiac adaptations in female athletes. They emphasize the need for gender-specific research in sports cardiology, as AHS in females can vary substantially from males due to differences in hormonal, physiological, and anthropometric factors (8).
From a clinical perspective, these results can help in the differentiation of physiological AHS from pathological conditions, particularly in female athletes who might present with remodeling patterns mimicking earlier manifestations of cardiomyopathies (8, 18). Additionally, the observed relationship between WTT and cardiac chamber indices highlights the importance of monitoring training loads to optimize athlete performance while minimizing potential risks of overtraining (19).
Study limitations
The cross-sectional design precludes causal inferences, and the small sample size limits the extrapolation of the findings. Additionally, the lack of sedentary controls and the absence of detailed data on training programs restrict the robustness of these TTE findings. Future longitudinal studies with larger samples and detailed sports-associated parameters with complex regression models are needed to validate these findings and explore potential long-term implications of AHS in female athletes.
Conclusion
In summary, this study demonstrates that endurance training is associated with greater cardiac remodeling effects in young female athletes compared to mixed training, with WTT playing a significant role in these adaptations. The findings underscore the importance of tailored training strategies and the need for further research into gender-specific cardiac remodeling patterns in athletes.
Ethics: The study was approved by the Institutional Review Board of the Ala-Too International University. All participants provided informed consent before participating in the study. Data was collected anonymously and stored securely to ensure confidentiality.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: D.A., A.B., F.K., S.P., A.P., A.A., K.O., R.K., and T.K. equally contributed to manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Chat GPT 4.0 was used in case of manuscript writing. Authors checked the A.I. generated text and carry full responsibility for correctness.
Availability of data and material: Not applied
References
| 1.Martinez MW, Kim JH, Shah AB, Phelan D, Emery MS, Wasfy MM, et al. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease. J Am Coll Cardiol 2021; 78: 1453-70. doi: 10.1016/j.jacc.2021.08.003. PMID: 34593128. https://doi.org/10.1016/j.jacc.2021.08.003 PMid:34593128 |
||||
| 2.Palermi S, Cavarretta E, D'Ascenzi F, Castelleti S, Ricci F, Vecchiato M, et al. Athlete's heart: A cardiovascular step-by-step multimodality approach. Rev Cardiovasc Med 2023; 24: 151. Doi: 10.31083/j.rcm2405151 https://doi.org/10.31083/j.rcm2405151 PMid:39076743 PMCid:PMC11273059 |
||||
| 3.Lewis EJ, McKillop A, Banks L. The Morganroth hypothesis revisited: endurance exercise elicits eccentric hypertrophy of the heart. J Physiol 2012; 590: 2833-4. doi: 10.1113/jphysiol.2011.226217 https://doi.org/10.1113/jphysiol.2011.226217 PMid:22707591 PMCid:PMC3448147 |
||||
| 4.Spence AL, Naylor LH, Carter HH, Buck CL, Dembo L, Murray CP, et al. A prospective randomised longitudinal MRI study of left ventricular adaptation to endurance and resistance exercise training in humans. J Physiol 2011; 89: 5443-52. doi: 10.1113/jphysiol.2011.217125 https://doi.org/10.1113/jphysiol.2011.217125 PMid:21969450 PMCid:PMC3240883 |
||||
| 5.Rao P, Sharma A, Malhotra A. Defining normal cardiac adaptations in mixed-race athletes and the utility of race to categorize athletes (Internet). Expert Analysis 2021. Available at: URL: https://www.acc.org | ||||
| 6.Francavilla CV, Sessa F, Salerno M, Albano GD, Villano I, Messina G, et al. Influence of football on physiological cardiac indexes in professional and young athletes. Front Physiol 2018; 9: 153. doi: 10.3389/fphys.2018.00153 https://doi.org/10.3389/fphys.2018.00153 PMid:29541036 PMCid:PMC5835836 |
||||
| 7.Bassareo PP, Crisafulli A. Gender differences in hemodynamic regulation and cardiovascular adaptations to dynamic exercise. Curr Cardiol Rev 2020; 16: 65-72. doi: 10.2174/1573403X15666190321141856. https://doi.org/10.2174/1573403X15666190321141856 PMid:30907327 PMCid:PMC7393595 |
||||
| 8.Colombo CSSS, Finocchiaro G. The female athlete's heart: facts and fallacies. Curr Treat Options Cardiovasc Med 2018; 20: 101. doi: 10.1007/s11936-018-0699-7 https://doi.org/10.1007/s11936-018-0699-7 PMid:30390143 PMCid:PMC6223714 |
||||
| 9.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease: The Task Force on sports cardiology and exercise in patients with cardiovascular disease of the European Society of Cardiology (ESC). Eur Heart J 2021; 42: 17-96. doi: 10.1093/eurheartj/ehaa605 https://doi.org/10.1093/eurheartj/ehaa605 PMid:32860412 |
||||
| 10.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernando L, et al. Echocardiography. The European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 412. doi: 10.1093/ehjci/jew041 https://doi.org/10.1093/ehjci/jew041 PMid:26983884 |
||||
| 11.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010; 23: 465-95; doi: 10.1016/j.echo.2010.03.019 https://doi.org/10.1016/j.echo.2010.03.019 PMid:20451803 |
||||
| 12.Nyberg J, Jakobsen EO, Østvik A, Holte E, Stølen S, Lovstakken L, et al. Echocardiographic reference ranges of global longitudinal strain for all cardiac chambers using guideline-directed dedicated views. JACC Cardiovasc Imaging 2023; 16: 1516-31. doi: 10.1016/j.jcmg.2023.08.011 https://doi.org/10.1016/j.jcmg.2023.08.011 PMid:37921718 |
||||
| 13.Brown B, Somauroo J, Green DJ, Wilson M, Drezner J, George K, Oxborough D. The complex phenotype of the athlete's heart: Implications for preparticipation screening. Exerc Sport Sci Rev 2017; 45: 96-104. doi: 10.1249/JES.0000000000000102 https://doi.org/10.1249/JES.0000000000000102 PMid:28306678 |
||||
| 14.Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation 2011; 123. doi: 10.1161/CIRCULATIONAHA.110.981571 https://doi.org/10.1161/CIRCULATIONAHA.110.981571 PMid:21670241 |
||||
| 15.Mitchell J, Haskell W, Snell P, Van SP. Task Force 8: Classification of sports. J Am Coll CArdiol 2005; 45: 1364-67. doi: 10.1016/j.jacc.2005.02.015 https://doi.org/10.1016/j.jacc.2005.02.015 PMid:15837288 |
||||
| 16. Doronina A, Édes IF, Ujvári A, Kántor Z, Lakatos BK, Tokodi M, et al. The Female athlete's heart: comparison of cardiac changes induced by different types of exercise training using 3d echocardiography. Biomed Res Int 2018; 2018: 3561962. doi: 10.1155/2018/3561962 https://doi.org/10.1155/2018/3561962 PMid:29998132 PMCid:PMC5994567 |
||||
| 17. Halson SL. Monitoring training load to understand fatigue in athletes. Sports Med 2014; 44 Suppl 2: S139-47. doi: 10.1007/s40279-014-0253-z https://doi.org/10.1007/s40279-014-0253-z PMid:25200666 PMCid:PMC4213373 |
||||
| 18.Maron BJ. Sudden death in young athletes. N Engl J Med 2003; 349: 1064-75. doi: 10.1056/NEJMra022783 https://doi.org/10.1056/NEJMra022783 PMid:12968091 |
||||
| 19.Eijsvogels TMH, Thompson PD, Franklin BA. The "Extreme Exercise Hypothesis": Recent findings and cardiovascular health implications. Curr Treat Options Cardiovasc Med 2018; 20: 84. doi: 10.1007/s11936-018-0674-3 https://doi.org/10.1007/s11936-018-0674-3 PMid:30155804 PMCid:PMC6132728 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

