Atypical acute myocardial infarction presenting as papillary muscle rupture and pulmonary edema: A case report
CASE REPORT
Atypical acute myocardial infarction presenting as papillary muscle rupture and pulmonary edema: A case report
Article Summary
- DOI: 10.24969/hvt.2024.545
- CARDIOVASCULAR DISEASES
- Published: 15/02/2025
- Received: 26/10/2024
- Revised: 09/01/2025
- Accepted: 10/01/2025
- Views: 4976
- Downloads: 1996
- Keywords: Acute myocardial infarction, acute mitral regurgitation, transthoracic echocardiography, papillary muscle rupture, heart failure, ischemia, case report
Address for Correspondence: Irina A. Akhmedova, Higher International School of Medicine, Scientific Research Institute Cardiac Surgery and Organ Transplantation, Bishkek, Kyrgyzstan
Email: akhmedovairina1@gmail.com
ORCID: Irina A. Akhmedova – 0000-0002-5438-2441; Azat K. Turgunov- 0009-0002-5438-2441
Irina A. Akhmedova,1,2, Azat K. Turgunov1,2, Inamullah1, Areeba Sarfraz1, Rakhat S. Kalieva2, Sultanmurat A. Jumabaev2, Daniyar Ch.Cholponbaev1,2
1 Higher International School of Medicine, Bishkek, Kyrgyzstan
2Scientific Research Institute Cardiac Surgery and Organ Transplantation, Bishkek, Kyrgyzstan
Abstract
Objective: We present a rare and diagnostically challenging case of rupture of the posteromedial papillary muscle caused by chronic ischemia of the circumflex artery. The uniqueness of this case lies in the absence of typical symptoms of acute myocardial infarction (AMI), including chest pain, as well as atypical electrocardiographic findings that do not indicate AMI.
Case presentation: A 70-year-old female with hypertension presented with progressive dyspnea but no chest pain or AMI signs on electrocardiogram. Initial heart failure treatment yielded no improvement, prompting echocardiography, which revealed prolapse of the posterior mitral leaflet and free movement of the posteromedial papillary muscle, resulting in acute mitral regurgitation. Coronary angiography confirmed multivessel coronary artery disease, with significant stenosis in the circumflex artery. The patient underwent mitral valve replacement with coronary artery bypass grafting.
Conclusion: This case highlights the potential for atypical presentations of papillary muscle rupture in chronic ischemic conditions and underscores the importance of echocardiography in cases with ambiguous clinical findings.
Key words: Acute myocardial infarction, acute mitral regurgitation, transthoracic echocardiography, papillary muscle rupture, heart failure, ischemia, case report
Introduction
The most common mechanical complications of acute myocardial infarction (AMI) include acute mitral regurgitation (MR) secondary to papillary muscle rupture, ventricular septal defect, pseudo aneurysm, and free wall rupture (1). The development of severe MR complicating AMI and leading to cardiogenic shock (CS_ is widely recognized as a medical catastrophe, indicating a very poor prognosis (2). Papillary muscle rupture is a rare, life-threatening complication of myocardial infarction, accompanied by significant hemodynamic disturbances and the development of severe acute heart failure. It occurs at a frequency of 0.029%, more commonly in men—60.4%, with an age of ≥65 years—60.1% (3). It is associated with a relatively high mortality rate. According to several studies of hospitalizations with mechanical complications of AMI, in-hospital mortality reaches 42.2% (2, 4, 5). Medical literature describes isolated cases of papillary muscle rupture without AMI, in the context of multivessel coronary artery disease, resulting from chronic myocardial ischemia.
In this case, we aim to present a case of mitral valve papillary muscle rupture in a patient with chronic ischemic multivessel coronary artery disease and concomitant arterial hypertension.
Case report
In this case, we present a case of rupture of the mitral valve papillary muscle in a patient with chronic ischemic multivessel coronary artery disease and concomitant arterial hypertension.
A 70-year-old woman with a history of hypertension was admitted to our clinic with severe shortness of breath and orthopnea. She did not complain of chest pain. The patient was hospitalized in a primary-level hospital in a remote area far from a medical center, in the general emergency unit, without the presence of specialized cardiologists before referral to our clinic. The patient's medical history did not indicate any heart murmurs or mitral valve prolapse. According to the patient, her condition worsened gradually over the course of a week, as her dyspnea increased. The patient also denied ever having experienced chest pain.
Her blood pressure upon admission was 140/90 mm Hg. Physical examination: On auscultation of the lungs – scattered bilateral fine crackles, no heart murmurs were detected.
Standard laboratory tests taken according to protocol were unremarkable. Given the patient's hospitalization in a remote area far from a medical center, tests for troponin and other cardiac biomarkers were unavailable.
The electrocardiogram (ECG) (Fig. 1) upon admission showed: normal heart axis, occasional ventricular and atrial extrasystoles, ST-segment depression in leads V2-V4, and T-wave inversion in V1-V4.
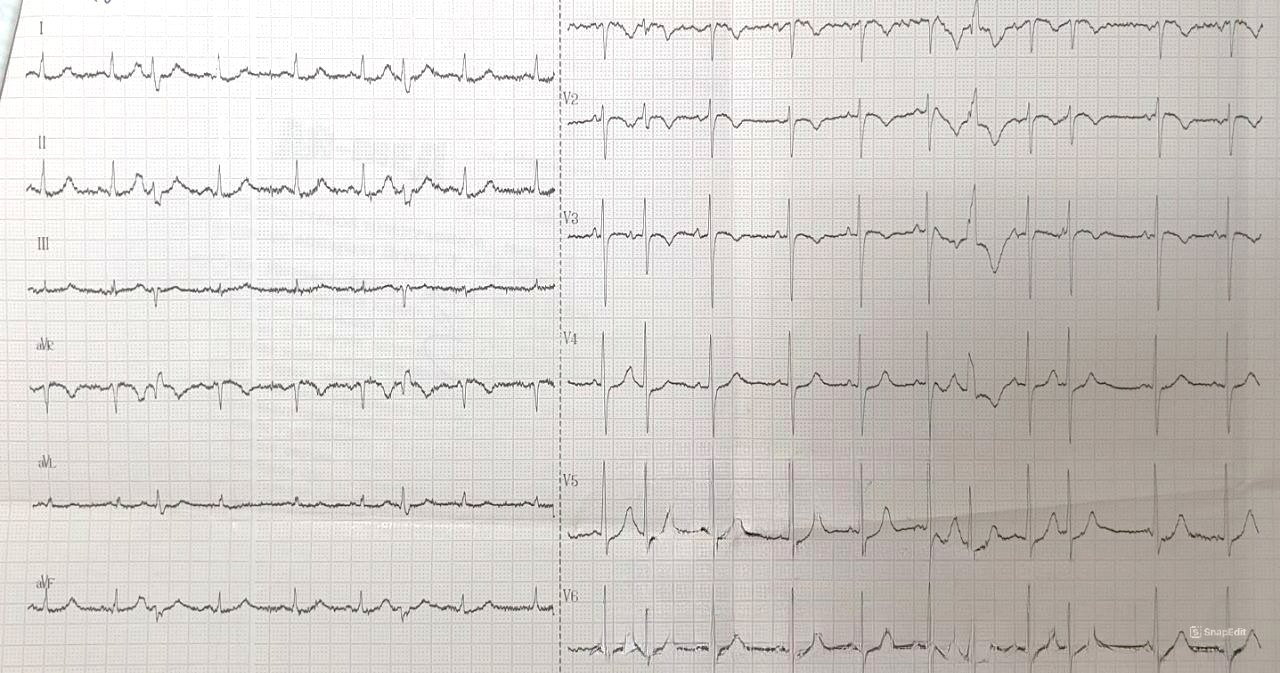
Figure 1. Electrocardiogram displays occasional ventricular and atrial extrasystoles, ST-segment depression in leads V2-V4, and T-wave inversion in V1-V4
A computed tomography (CT) scan was performed, which indicated interstitial pulmonary edema and bilateral pleural effusion (requiring differentiation from hypostatic pneumonia). The heart contours were of normal size.
Treatment following the acute heart failure protocol did not yield results. As a result, echocardiography was performed 21 days after the onset of heart failure symptoms, which revealed significant changes. Transthoracic echocardiography (TTE) demonstrated prolapse of the posterior leaflet and the free papillary muscle of the posterior leaflet into the left atrium (LA) (free movement of the posterior leaflet with the posteromedial papillary muscle (PMPM), with a complete lack of mitral valve leaflet coaptation) (Fig. 1, 2). TTE also revealed significant MR and a markedly increased LA. The contractility of the left ventricular (LV) myocardium was normal. LA diameter was 52 mm, LA volume – 161 ml, end-diastolic LV diameter – 51 mm, end-diastolic LV volume – 99 ml, end systolic LV volume – 33 ml, and LV ejection fraction (LVEF) of 60%, measured by Simpson’s biplane method from 4AC position. Pulmonary arterial pressure was increased - 100 mmHg. At the time of the patient's examination, transesophageal echocardiography was unavailable at the medical facility due to technical and organizational reasons.
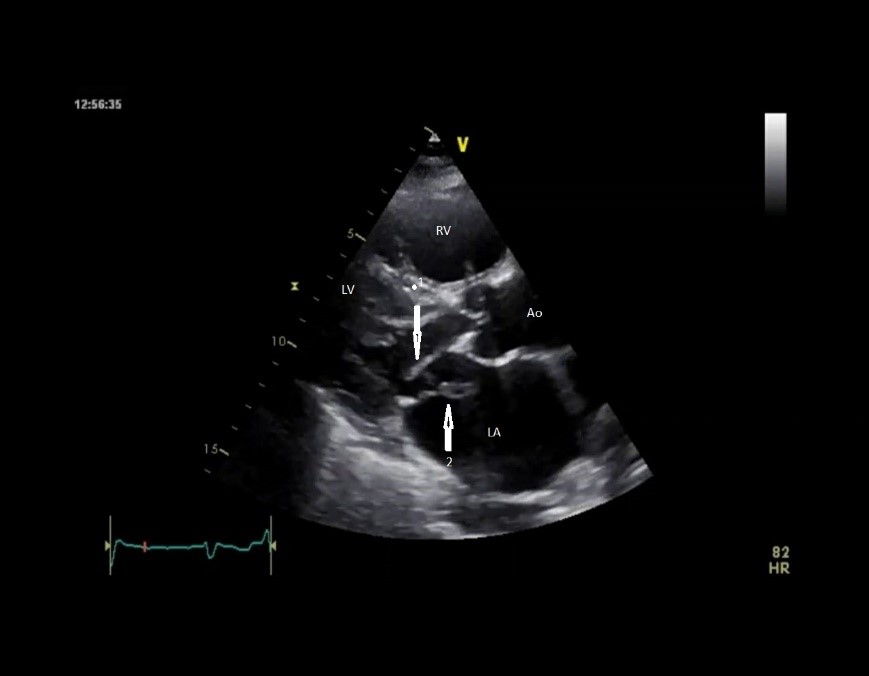
Figure 2. Parasternal long-axis view of the prolapse of the posterior leaflet and the free papillary muscle of the posterior leaflet into the left atrium (arrow)
Ao – aorta, LA - left atrium, LV - left ventricle, RV – right ventricle
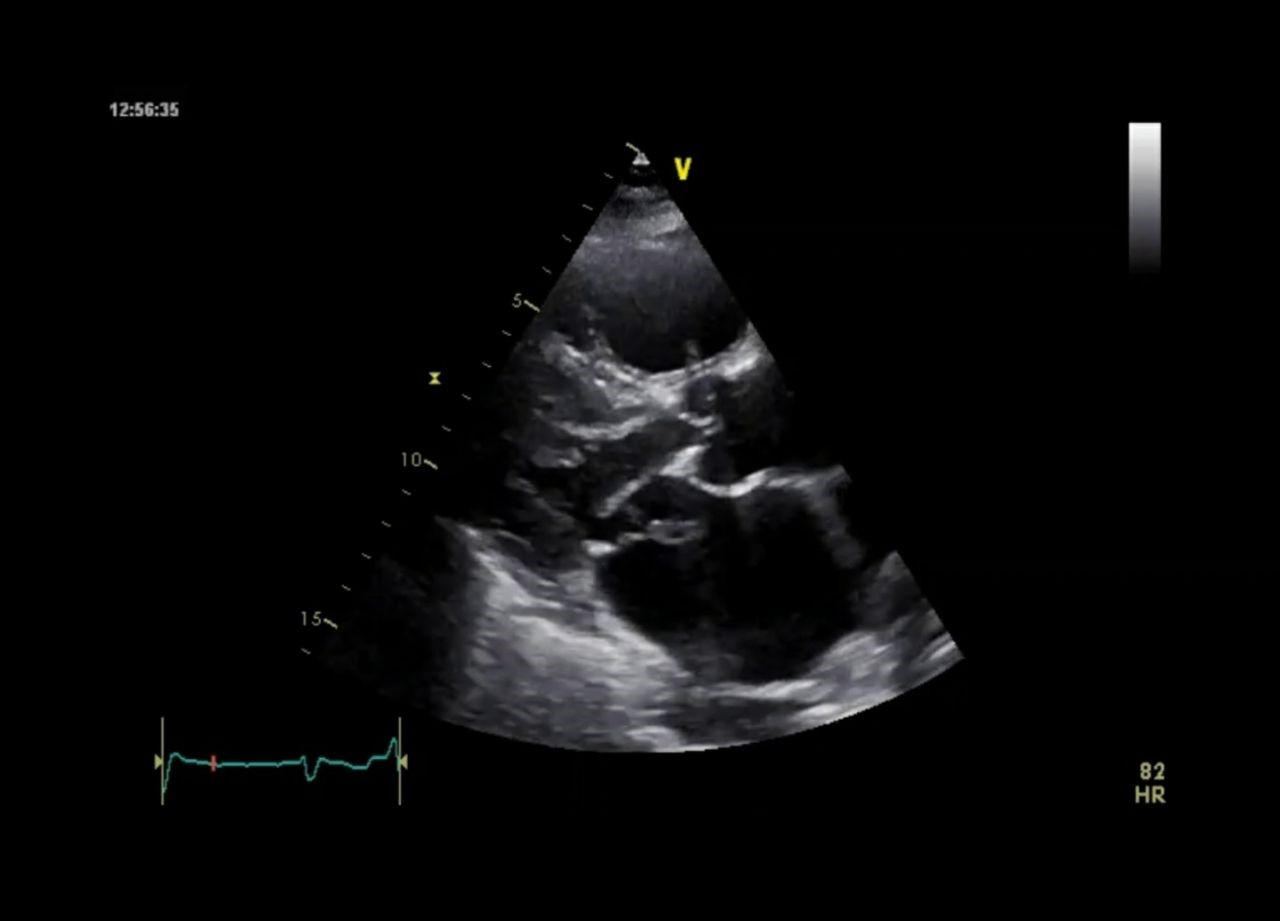
Figure 3. Parasternal long-axis view – prolapse of the posterior leaflet and the free papillary muscle of the posterior leaflet into the left atrium
Troponin levels showed a significant increase of 1.17 (study 21 days after the onset of symptoms). Emergency coronary angiography was performed (Fig. 4), revealing: left-dominant coronary circulation; significant mid-segment stenosis of the left circumflex artery (LCx), significant diffuse stenosis of the distal segment of the LCx and moderate mid-segment stenosis of the left anterior descending artery (LAD) and right coronary artery (RCA) without significant lesions.

Figure 4. A – Significant mid-segment stenosis of the circumflex artery (white arrow), significant diffuse stenosis of the distal segment of the circumflex artery (blue arrow). B - Significant mid-segment stenosis of the circumflex artery (white arrow). Moderate mid-segment stenosis of the left anterior descending artery (red arrow). C - Moderate mid-segment stenosis of the left anterior descending artery (red arrow). D – The right coronary artery without significant lesions
A decision was made to perform mitral valve replacement (MVR) combined with coronary artery bypass grafting (CABG), as restoring the ischemic papillary muscle along with mitral-annular continuity could have led to variable outcomes.
The patient successfully underwent cardiac surgery under cardiopulmonary bypass, with MVR using a mechanical, two-disc "St. Jude-29" prosthesis, and bypass grafting of the circumflex artery, its obtuse marginal (OM) branch, LAD and its diagonal (D1) branch. During the revision of the mitral valve, rupture of the PMPM was confirmed. Pathological examination of the ruptured PMPM revealed extensive necrosis.
The postoperative period was stable. The patient was weaned off mechanical ventilation after 12 hours and remained in the intensive care unit for 2 days. The patient was successfully discharged from the hospital on the 10th day.
Discussion
Papillary muscle rupture is a serious, life-threatening complication of AMI (6-9). In most cases, this complication occurs in patients with AMI, and rare cases of papillary muscle rupture in patients with chronic coronary artery occlusion have been documented. Rupture of the PMPM occurs 6–12 times more frequently than rupture of the anterolateral papillary muscle. While the anterolateral papillary muscle has dual blood supply from the LAD and the LCx, the PMPM has a single blood supply from the RCA or the LCx (8, 9).
In typical conditions, the LCx primarily supplies the anterolateral papillary muscle, while the posteromedial papillary muscle predominantly depends on blood supply from the RCA. However, in patients with left coronary dominance, as identified in this case, the posteromedial papillary muscle receives its blood supply predominantly through the LCx (10).
Retrospectively analyzing the patient's history, it becomes clear that when acute symptoms appeared—such as shortness of breath at rest and air hunger—the patient was hospitalized in a primary-level hospital in a remote area, where there were no resident cardiologists or TTE available. It is important to consider that we are living in the post-COVID period, and the patient's general condition, complaints, and the absence of typical signs of myocardial infarction were interpreted by the medical staff as primary lung damage. As a result, the primary diagnostic choice was a chest CT scan, rather than TTE. The CT report showed evidence of interstitial lung edema and bilateral pleural effusion (with the need to differentiate from hypostatic pneumonia). The heart contours appeared normal in size. The CT findings were interpreted as confirming the primary diagnosis—lung damage, which, as we can retrospectively assess, was an incorrect diagnosis. The development of acute heart failure and pulmonary edema in this patient, associated with acute, total mitral valve insufficiency, was initially masked by the presence of pulmonary sounds on auscultation, which obscured the auscultatory findings of severe MR. Additionally, the patient's electrocardiogram did not show typical signs of AMI. It was only on the 20th day, following a worsening of the patient's condition during pneumonia treatment, that a TTE was performed by an external specialist, who was unable to properly interpret the results and sent them for evaluation to an echocardiography physician at the leading cardiovascular center in the country. After reviewing the echocardiographic data in consultation with the lead cardiologist, the patient was urgently transferred to the leading cardiothoracic center, where an urgent repeat TTE, coronary angiography, and troponin levels were measured. The lack of dynamic data from biomarkers and the ECG complicated the final exclusion of classical myocardial infarction. The elevated troponin levels were believed to be the result of papillary muscle damage, and the coronary angiography revealed multivessel coronary artery disease. The patient was then transferred to the cardiothoracic team for combined surgery: MVR and CABG.
Analyzing the patient's medical history and status in the emergency unit, we hypothesize that, against the background of elevated blood pressure and multivessel ischemic coronary artery disease, an atypical variant of AMI occurred, with rupture of the posteromedial papillary muscle, without the typical signs of AMI on the ECG and without a reduction in the LVEF, as demonstrated by TTE.
Retrospectively, by carefully analyzing the ECG in light of additional information, such as coronary angiography and troponin levels, we arrived at the following conclusions:
1.ST-segment depression in leads V2-V4 could be interpreted as a sign of possible ischemia or a past myocardial infarction.
2.T-wave inversion is noted in chest leads V2-V4, which can be interpreted as a sign of ischemic changes in the myocardium.
3.The QRS complexes are mostly of normal width. Changes in the shape of QRS complexes are observed in chest leads (V2-V4), which can be interpreted as pathological changes in the myocardium.
The preservation of myocardial contractility, as demonstrated on echocardiography, was likely one of the factors that contributed to the favorable outcome in this case, considering the duration from the onset of symptoms to the cardiac surgery performed.
Conclusion
We believe that our case is rare, with an atypical course of posteromedial papillary muscle rupture associated with an atypical variant of AMI, linked to chronic damage of the circumflex coronary artery. The absence of a typical clinical picture and the atypical ECG findings (ST depression, T wave inversion) can be explained by the peculiarities of blood supply to the posteromedial papillary muscle and the characteristics of left coronary dominance. The lack of dynamic data on biomarkers and the absence of typical signs of myocardial infarction on the ECG complicated the establishment of a timely and accurate diagnosis. Therefore, we believe that this condition can be considered as an atypical AMI, occurring in the context of chronic ischemia and limited perfusion through the LCx, which led to a significant reduction in blood supply to the papillary muscle, resulting in ischemic necrosis and clinically manifested as acute pulmonary edema and severe mitral regurgitation.
Take-Home Message
- Rupture of the posteromedial papillary muscle can occur even in the absence of typical signs of acute myocardial infarction on the ECG.
- Accurate diagnosis requires timely performed echocardiography. Transesophageal echocardiography is preferred for differential diagnosis with infective endocarditis.
- A multidisciplinary approach involving a cardiologist, echocardiographer, and cardiothoracic surgeon is essential for successful outcomes.
- Surgical intervention, including mitral valve replacement and CABG, significantly improves the prognosis in such patients.
Ethics: Ethical approval was not required for this single-case study in accordance with the policies of Research Institute of Heart Surgery and Organ Transplantation, as it did not involve experimental interventions. Written informed consent was obtained from the patient for the publication of this case report and accompanying images. All identifying details have been removed to ensure the patient's privacy.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: I.A.A., A.K.T., I., A.S., R.S.K., S.A.J., and D.Ch.Ch. equally contributed to case management, manuscript preparation and fulfilled the authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use: Authors declared they did not use A.I.- assisted technologies in preparation of manuscript
Availability of data and material: Do not apply
References
| 1.Damluji AA, van Diepen S, Katz JN, Menon V, Tamis-Holland JE, Bakitas M, et al.J; American Heart Association Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Surgery and Anesthesia; and Council on Cardiovascular and Stroke Nursing. Mechanical complications of acute myocardial infarction: A Scientific Statement from the American Heart Association. Circulation 2021; 144: e16-e35. doi: 10.1161/CIR.0000000000000985. https://doi.org/10.1161/CIR.0000000000000985 |
||||
| 2.Thompson CR, Buller CE, Sleeper LA, Antonelli TA, Webb JG, Jaber WA, et al. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we use emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol 2000; 36(3 Suppl A): 1104-9. doi: 10.1016/s0735-1097(00)00846-9 https://doi.org/10.1016/S0735-1097(00)00846-9 PMid:10985712 |
||||
| 3.Bhardwaj B, Sidhu G, Balla S, Kumar V, Kumar A, Aggarwal K, et al.. Outcomes and hospital utilization in patients with papillary muscle rupture associated with acute myocardial infarction. Am J Cardiol 2020; 125: 1020-5. doi: 10.1016/j.amjcard.2019.12.051 https://doi.org/10.1016/j.amjcard.2019.12.051 PMid:31973809 |
||||
| 4.Kameyama A, Imamura H, Kamijo H, Takeshige K, Mochizuki K, Nitta K. Diagnostic pitfalls in papillary muscle rupture-associated acute mitral regurgitation after acute myocardial infarction. Case Rep Crit Care 2021; 21: 1396194 doi: 10.1155/2021/1396194 https://doi.org/10.1155/2021/1396194 PMid:34970457 PMCid:PMC8714394 |
||||
| 5.Elbadawi A, Elgendy IY, Mahmoud K, Barakat AF, Mentias A, Mohamed AH, et al. Temporal trends and outcomes of mechanical complications in patients with acute myocardial infarction. JACC Cardiovasc Interv 2019; 12: 1825-36. doi: 10.1016/j.jcin.2019.04.039 https://doi.org/10.1016/j.jcin.2019.04.039 PMid:31537282 |
||||
| 6.Porwal KH, Porwal MH, Ibrahim MM, Ramaswamykanive H, Gupta K, Mathur M, et al. Atypical presentation of acute mitral regurgitation secondary to papillary muscle rupture. Cureus 2022; 14: e24744. doi: 10.7759/cureus.24744 https://doi.org/10.7759/cureus.24744 |
||||
| 7.Hamid UI, Aksoy R, Sardari Nia P. Mitral valve repair in papillary muscle rupture. Ann Cardiothorac Surg 2022; 11: 281-9. doi: 10.21037/acs-2021-ami-23 https://doi.org/10.21037/acs-2021-ami-23 PMid:35733722 PMCid:PMC9207695 |
||||
| 8.Sawhney V, Murugan S, Iqbal F, Muthumala A. Rupture of mitral valve papillary muscle: a rare complication following myocardial infarction. BMJ Case Rep 2020; 13: e232626. doi: 10.1136/bcr-2019-232626 https://doi.org/10.1136/bcr-2019-232626 PMid:31948978 PMCid:PMC7021130 |
||||
| 9.Fujita T, Yamamoto H, Kobayashi J, Fukushima S, Miyata H, Yamashita K, et al. Mitral valve surgery for ischemic papillary muscle rupture: outcomes from the Japan cardiovascular surgery database. Gen Thorac Cardiovasc Surg 2020; 68: 1439-46. doi: 10.1007/s11748-020-01418-y https://doi.org/10.1007/s11748-020-01418-y PMid:32588291 PMCid:PMC7680308 |
||||
| 10.Stefanovski D, Walfisch A, Kedev S. Isolated right coronary lesion and anterolateral papillary muscle rupture - case report and review of the literature. J Cardiothorac Surg 2012; 1: 75. Doi: 10.1186/1749-8090-7-75 https://doi.org/10.1186/1749-8090-7-75 PMid:22898299 PMCid:PMC3441214 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

