Prevalence of atherosclerotic cardiovascular diseases and risk factors among pre-dialysis chronic kidney disease patients at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia: A cross-sectional study
ORIGINAL RESEARCH ARTICLE
Prevalence of atherosclerotic cardiovascular diseases and risk factors among pre-dialysis chronic kidney disease patients at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia: A cross-sectional study
Article Summary
- DOI: 10.24969/hvt.2025.563
- CARDIOVASCULAR DISEASES
- Published: 07/05/2025
- Received: 27/06/2024
- Revised: 17/04/2025
- Accepted: 18/04/2025
- Views: 3408
- Downloads: 1707
- Keywords: Chronic kidney disease, cardiovascular diseases, risk factors, pre-dialysis, Ethiopia
Address for Correspondence: Yidnekachew Asrat Birhan, Addis Ababa University, College of Health Sciences, School of Medicine, Department of Internal Medicine, Addis Ababa, Ethiopia
E-mail: yidnekachew.asrat@aau.edu.et
Yidnekachew Asrat Birhan*, Sintayehu Abebe Gebru, Addisu Melkie Ejigu, Dessalew Mekonnen, Tesfamariam Aklilu Betemariam, Senbeta Guteta Abdissa
Addis Ababa University, College of Health Sciences, School of Medicine, Department of Internal Medicine, Addis Ababa, Ethiopia
Abstract
Objective: Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among patients with chronic kidney disease (CKD). These patients have a higher prevalence of both traditional and non-traditional cardiovascular risk factors compared to the general population. Glomerular filtration rate (GFR) and proteinuria are independent predictors of cardiovascular outcomes. The objective of the study was to assess the prevalence of atherosclerotic cardiovascular diseases (ASCVD) and associated risk factors among pre-dialysis CKD patients at Tikur Anbessa Specialized Hospital.
Methods: A hospital-based cross-sectional study was conducted from April 5 to August 27, 2021, among 216 randomly selected pre-dialysis CKD patients attending renal clinics at Tikur Anbessa Specialized Hospital. Structured questionnaires were used to collect sociodemographic, clinical, laboratory, and imaging data. Data were anonymized, entered into Epi Info 3.1, and analyzed using SPSS version 25. Descriptive and multivariable logistic regression analyses were performed. Ethical clearance was obtained from the Institutional Review Board of the Department of Internal Medicine, Addis Ababa University.
Results: Out of 216 participants, 149 (69%) were male, with a mean age of 55.6 years (95% CI: 41.9–68.6). The leading causes of CKD were diabetes mellitus (41.7%) and hypertension (34.3%). The mean eGFR was 37.3 mL/min/1.73 m² (95% CI: 23.3–51.5). Hypertension and diabetes were prevalent in 91.2% and 48.1% of participants, respectively. Anemia and hypertriglyceridemia were each present in 36.8%. The prevalence of ischemic heart disease, cerebrovascular disease, and peripheral arterial disease were 14.4%, 11.1%, and 4.2%, respectively, resulting in an overall ASCVD prevalence of 27.8% (95% CI: 21.9–34.3). Left ventricular hypertrophy was present in 28.2%, diastolic dysfunction in 30.6%, and systolic dysfunction in 20.8%. Uncontrolled office blood pressure (AOR: 4.7; 95% CI: 1.17–18.7; p = 0.029) and khat chewing (AOR: 5.1; 95% CI: 1.10–24.66; p = 0.042) were significantly associated with ASCVD.
Conclusion: The burden of atherosclerotic cardiovascular disease among pre-dialysis CKD patients in this study was notably high. Hypertension, diabetes, anemia, and cardiac abnormalities were also highly prevalent. These findings highlight the need for early cardiovascular screening and risk factor management in this population.
Key words: Chronic kidney disease, cardiovascular diseases, risk factors, pre-dialysis, Ethiopia
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and premature mortality among patients with chronic
kidney disease (CKD). Since the early observations by Richard Bright, the strong association between CKD and CVD has been well established (1). Although the elevated cardiovascular (CV) risk in end-stage renal disease (ESRD) is widely recognized, increasing evidence shows that patients across the entire spectrum of CKD are at significantly heightened risk for cardiovascular events (1, 2).
|
Acronyms and abbreviations |
|
|
Abbreviation |
Definition |
|
ABI |
Ankle brachial index |
|
BSA |
Body surface area |
|
CKD |
Chronic kidney disease |
|
CKD-EPI |
Chronic kidney disease epidemiology collaboration |
|
CRIC |
Chronic renal insufficiency cohort |
|
CRISIS |
Chronic Renal Insufficiency Standards Implementation Study |
|
CVD |
Cerebrovascular disease |
|
eGFR |
Estimated glomerular filtration rate |
|
ESRD |
End stage renal disease |
|
HIV |
Human immunodeficiency virus |
|
IHD |
Ischemic heart disease |
|
iPTH |
Intact parathyroid hormone |
|
LVH |
Left ventricular hypertrophy |
|
PAD |
Peripheral arterial disease |
CKD patients exhibit a high prevalence of both traditional and non-traditional CV risk factors. Traditional risk factors such as hypertension, diabetes, dyslipidemia, smoking, obesity, and older age are common in both the general population and in those with CKD. In contrast, CKD-related (non-traditional) risk factors—including anemia, calcium-phosphorus imbalance, chronic inflammation, oxidative stress, hypoalbuminemia, and left ventricular hypertrophy—contribute further to the excess CV risk observed in this population (3, 4).
Both estimated glomerular filtration rate (eGFR) and albuminuria have been identified as independent, graded predictors of CV morbidity and mortality. CKD is now considered a cardiovascular risk equivalent, comparable to diabetes mellitus and coronary heart disease (5, 6).
Evidence from large community-based studies involving over 20,000 participants confirms that CKD is an independent risk factor for myocardial infarction, stroke, and CV death, even after excluding individuals with known baseline CVD (5). Notably, individuals of African descent appear to be at higher cardiovascular risk than other populations. Reported CVD prevalence among CKD cohorts includes 26.8% in CKD-ROUTE (Japan), 33.4% in CRIC (U.S.), 47.2% in CRISIS (U.K.), 39.1% in MERENA (Spain), and 9.8% in C-STRIDE (China) (7–11).
In Africa, limited data from observational studies also suggest a high burden of CVD among CKD patients. A study from South Africa reported a 47.5% prevalence of atherosclerotic vascular disease in CKD patients, while a Nigerian study found electrocardiographic abnormalities in 86%, diastolic dysfunction in 62.8%, and systolic dysfunction in 15.1% of patients. Similarly, a Ugandan study documented left ventricular hypertrophy (LVH) in 54.4%, systolic dysfunction in 18.9%, and diastolic dysfunction in 17.5% of pre-dialysis CKD patients (12–16).
A systematic review and meta-analysis further highlighted that male gender, advancing age, smoking, diabetes, established CVD, and elevated total cholesterol were associated with increased CV risk in CKD patients. In addition, non-traditional risk factors such as anemia, hypoalbuminemia, hyperphosphatemia, and hyperuricemia were significantly linked to higher rates of CVD events (17, 18).
A Ugandan study of 217 CKD patients demonstrated high prevalence rates of hypertension (90%), anemia (71.9%), hypocalcemia (44.7%), hyperphosphatemia (39.2%), diabetes (16.1%), obesity (10.2%), and smoking (11.5%), with these risks increasing in prevalence and severity as renal function declined. The same study also found that HIV prevalence among CKD patients was approximately twice that of the general population (13).
The cumulative effect of traditional and non-traditional risk factors accelerates atherosclerosis and worsens CKD progression (17). Despite these findings from other settings, there remains a lack of data from Ethiopia, and only a few small studies have been conducted in similar resource-limited environments.
The aim of this study was to assess the prevalence and associated risk factors of atherosclerotic cardiovascular disease among pre-dialysis CKD patients at Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia.
Methods
Study design and population
A cross-sectional study design was used.
Study area and study period
We conducted this study over five month’s period from April 5 to August 27, 2021 at Tikur Anbessa Specialized Hospital (TASH), Addis Ababa. Addis Ababa is the capital city of Ethiopia. TASH is the country’s largest teaching and tertiary hospital with more than 400,000 patients seen at an outpatient follow-up clinic annually. About 300 patients are screened at the renal clinic every month with three clinics weekly and CKD accounts for more than a quarter of these patients.
Population
Source Population
All pre-dialysis CKD patients on follow-up at the renal clinic at TASH were the source population.
Study Population
All randomly selected pre-dialysis CKD patients on follow-up at renal clinics at TASH were the study population.
Eligibility Criteria
Inclusion criteria: All pre-dialysis CKD patients on follow up at the renal clinic at TASH who fulfill the following eligibility criteria were included. These were: Men or women aged between 18 and 74 years, those who have specified estimated GFR and who had follow-up at TASH for more than 6 months.
Exclusion criteria: We excluded individuals who were very ill and unable to consent, individuals who were on dialysis and post renal transplant patient.
Sampling technique
Sampling was done by systematic random sampling. The total number of pre-dialysis CKD patients having follow-up at TASH was retrieved from HMIS data and simple random sampling was done.
Ethical approval
Data collection was carried out after approval of the research proposal by institutional review board of Department of Internal Medicine, School of Medicine, College of Health Sciences, Addis Ababa University. Appropriate measures were taken to protect confidentiality of the collected information. All collected data didn’t contain patient identifications like names, patient’s medical record number and residence addresses. Written informed consent was taken from eligible patients. Patients were provided with information on the objectives of the study and confidentiality issues. At the end of the session, patients were given the opportunity to enroll in the study at that time and continued the appropriate CKD care per Ethiopian Federal Ministry of Health and international standard of care guidelines. Those who want to discuss their participation in the study with family members were given an opportunity to do.
Data collection method
Sociodemographic data (age, sex, urban or rural residency, educational status -no formal education, primary school, high school, diploma degree and above) was collected by interview, chart review and electronic record review.
CKD related facts, traditional and novel risk factors, medications and some of the CVDs were retrieved by interview, and chart and electronic record review.
Trained physicians collected those data. In assessing the CVDs, cardiology fellows did echocardiography and trained medical interns did lower extremity Doppler ultrasonography.
Anthropometric and clinical data included body mass index (BMI), waist and hip circumferences, a mean waist-to-hip ratio, and ankle brachial index (ABI) and blood pressure.
Among laboratory analysis the following parameters were assessed: hemoglobin and hematocrit, total cholesterol, low density lipoprotein cholesterol (LDL), high density lipoprotein cholesterol (HDL), and triglycerides; fasting blood glucose (FBG) and hemoglobin A1c (HbA1c); intact parathyroid hormone (iPTH); serum calcium and phosphorus, serum uric acid and albumin; erythrocyte sedimentation rate (ESR), creatinine and estimated glomerular filtration rate (eGFR), 24-hour urinary protein excretion, and serostatus for HIV.
For CKD causes, we assessed presence of diabetes mellitus (DM), hypertension, obstructive uropathy, glomerulonephritis and autosomal dominant polycystic kidney disease (ADPKD). We evaluated treatment of DM and hypertension, and mineral and bone disease.
We determined behavioral and family history risk factors as smoking status (ex-smoker, never smoker, and current smoker), family history of CVD, active chewing chat, active alcohol drinking, physical exercise and menopause status in females.
CVDs included - ischemic heart disease (IHD), cerebrovascular disease (ischemic stroke, hemorrhagic stroke, transient ischemic attack, TIA), and peripheral arterial disease (PAD). We evaluated treatment of CVDs. Echocardiography was used to determine LVH, diastolic dysfunction, systolic dysfunction, pulmonary hypertension and left ventricular ejection fraction.
Variables
Outcome variables
- Prevalence of atherosclerotic CVD (ASCVD) in pre-dialysis CKD patients
-Prevalence of CVD risk factors in pre-dialysis CKD patients
Explanatory variables
- Specific CVD
- Contribution of novel and traditional risk factors and their control
- Specific causes of CKD
- Stage of CKD
Operational Definitions
CKD was defined as a decreased eGFR to <60 ml/min/m2 of body surface area (bsa).
eGFR was calculated based on serum creatinine with CKD-EPI equation:
(eGFR = 141 * min(Scr/κ,1)α * max(Scr/κ, 1)-1.209 * 0.993Age * 1.018 [if female] * 1.159 [if black]).
ASCVD included: Ischemic heart disease (echocardiography evidence or history of acute coronary syndrome), peripheral arterial disease (history of documented peripheral arterial disease), or cerebrovascular events (history of ischemic or hemorrhagic stroke or transient ischemic attack).
CKD staging was based on eGFR (Stages 3a, 3b, and 4: 45-59, 30-44, and 15-29 ml/min/1.72m2 of BSA).
Traditional risk factors: risk factors established to increase the risk of ASCVD, which includes diabetes mellitus, hypertension, dyslipidemia, smoking, obesity, and family history.
Novel risk factors: were additional risk factors which are suspected culprits in both the development and progression of athero-thrombosis including CKD specific factors which includes anemia, albuminuria, hypoalbuminemia, increased inflammatory markers, and mineral bone disease.
Data quality control
The principal investigator undertook regular checkups of data collection methods and study protocol adherence. Regular checkup for completeness and consistency of the collected data was done. Two medical interns as data collectors were trained on the objectives of the study, interview technique, and data collection process. Cardiology fellows did echocardiography by standardized ultrasound machine (Philips). For office blood pressure measurement, (Sphygmomanometer- aneroid, China) and ankle brachial index measurement (hand held vascular Doppler- Lifedope L150R, India) internationally validated devices were used.
Statistical analysis
Sample size determination
The sample size is calculated using the single proportion formula
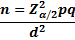
Z α/2 = is standard normal variant (at 5% type 1 Error (P <0.05) it is 1.96
d = margin of error was taken as 0.05.
p = expected proportion of the population with the event of outcome (prevalence) –the prevalence of CVD in CKD patients in a similar set up is 39.1%(13).
q =1-p: the probability of non-occurrence of the event of interest.
The calculated sample size was 366 patients.
Since the population size is less than 10,000, we made additional correction with
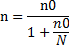
n= Final sample size
n0= Initial sample size (366)
N= Source population size (600)
The calculated sample size is 227 patients.
Data was checked for completeness, edited, coded and entered into Epi data version 3.1 and exported to SPSS version 25.0 statistical software for cleaning and analysis.
Frequencies and proportion were used to describe study subjects and socio-demographic characteristics. Continuous variables were expressed as means (standard deviation). Differences between group means were tested using two-tailed Student's t-test. Proportions were reported as percentages and compared between groups with Chi-square test. Pearson correlation analysis was used for evaluation of correlation between variables and CVD, while multiple logistic regression analysis was employed to establish determinative risk factors for CVD in patients with CKD.
A p value of less 0.05 was considered statistically significant.
Results
A total of 216 participants were included in this study, resulting in a response rate of 95%. Forty-one individuals were excluded for various reasons, as outlined in Figure 1. Nearly half of the participants (47.2%) were aged 60 years or older, with a mean age of 55.6 years (95% CI: 41.9–68.6). The majority were male, accounting for 69% (n = 149) of the study population. In terms of educational status, 12.5% (n = 27) had no formal education, while 31.5% (n = 68) had completed primary education, 28.7% (n = 62) had completed high school, 14.8% (n = 32) held a diploma, and 12.5% (n = 27) had a university degree. The detailed sociodemographic profile of the participants is summarized in Table 1.
Clinical and Laboratory Parameter Characteristics
In this study, the most common underlying cause of CKD was DM, accounting for 41.7% of cases, followed by hypertension (34.3%), obstructive uropathy (8.3%), glomerulonephritis (6%), and autosomal dominant polycystic kidney disease (ADPKD) (2.3%) (Table 2).
The mean estimated glomerular filtration rate (eGFR) was 37.3 mL/min/1.73 m² (95% CI: 23.3–51.5), and the median 24-hour urinary protein excretion was 745 mg (IQR: 342.2–1705.5).
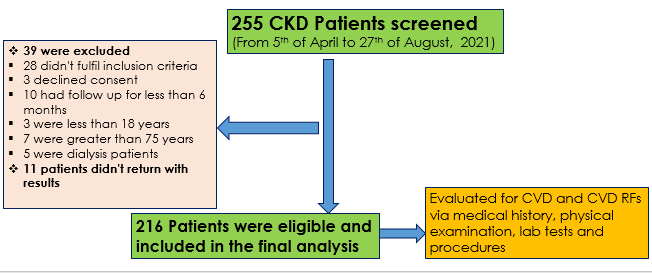
Figure 1. Participant`s flow chart showing number of patients screened, excluded and recruited in the study
CKD – chronic kidney disease, CVD – cardiovascular disease
|
Table 1. The sociodemographic characteristics of CKD patients at TASH renal clinic, Addis Ababa, Ethiopia, 2021 |
||
|
Variables |
Frequency (n=216) |
Percentage (%) |
|
Age |
|
|
|
≤29 years |
11 |
5.1 |
|
30-39 years |
23 |
10.6 |
|
40-49 years |
32 |
14.8 |
|
50-59 years |
48 |
22.2 |
|
≥60 years |
102 |
47.2 |
|
Gender |
|
|
|
Male |
149 |
69.0 |
|
Educational status |
|
|
|
No formal education |
27 |
12.5 |
|
Primary School |
68 |
31.5 |
|
High school |
62 |
28.7 |
|
Diploma |
32 |
14.8 |
|
Degree and above |
27 |
12.5 |
|
Residency area |
|
|
|
Urban |
129 |
59.7 |
|
Rural |
87 |
40.3 |
|
CKD – chronic kidney disease, TASH -Tikur Anbessa Specialized Hospital |
||
A total of 104 participants (48.1%) had DM. Among them, 71.1% were on insulin, while 29.7%, 10.6%, 4.8%, and 1% were
using metformin, sulfonylureas, sodium-glucose co-transporter 2 (SGLT2) inhibitors, and dipeptydil peptidase 4 (DPP-4) inhibitors, respectively. The mean FBS was 129.7 mg/dL (95% CI: 79.2–179.7), and the mean HbA1c level was 7.4% (95% CI: 5.2–9.6).
Hypertension was present in 197 participants (91.2%). The mean office systolic and diastolic blood pressures were 137.6 mmHg (95% CI: 117.5–157.7) and 75.5 mmHg (95% CI: 63.5–87.5), respectively. Calcium channel blockers were the most frequently prescribed antihypertensive agents, used by 80.2% of hypertensive patients. Renin-angiotensin-aldosterone system blockers (angiotensin converting enzyme inhibitors or angiotensin receptor blockers) were used by 67%, diuretics by 53.3%, and beta-blockers by 35.5%.
Regarding lipid profile, the mean total cholesterol, LDL, HDL, and triglyceride levels were 159.8 mg/dL (95% CI: 105.6–214), 105.0 mg/dL (95% CI: 63.8–146.2), 45.1 mg/dL (95% CI: 29.5–60.1), and 152.6 mg/dL (95% CI: 59–245.6), respectively. Statins were used by 120 participants (55.6%). The most common lipid abnormality was hypertriglyceridemia (36.8%), followed by low HDL (22.7%) and hypercholesterolemia (18.5%). Nearly half of the participants (49.1%) had LDL levels below 100 mg/dL, while only 4.3% had levels exceeding 190 mg/dL.
The mean hemoglobin and hematocrit levels were 13.4 g/dL (95% CI: 11–15.8) and 39.4% (95% CI: 32.6–45.4), respectively. Anemia, defined as hemoglobin <12 g/dL, was present in 30.6% of participants. Only 16 individuals (7.4%) were receiving treatment for anemia, all of whom were on oral iron; just one patient was on erythropoietin.
The median iPTH level was 94 IU/mL (IQR: 10–276). The mean serum calcium and phosphorus levels were 8.7 mg/dL (95% CI: 7.4–10) and 4.8 mg/dL (95% CI: 4.3–5.3), respectively. Treatment for mineral and bone disorder was documented in 22 participants (10.2%), with calcium and vitamin D supplements used in 19 (8.8%) and 7 (3.2%) participants, respectively.
The mean serum uric acid and albumin levels were 7.7 mg/dL (95% CI: 5.5–9.9) and 4.0 g/dL (95% CI: 3.5–4.5), respectively. The median erythrocyte sedimentation rate was 43 mm/hr (IQR: 28–70).
Physical measures and behavioral risk factors
Of the 216 participants, the majority (87%, n = 188) had never smoked, while 22 (10.2%) were former smokers and 6 (2.8%) were current smokers (Table 3). Among ever-smokers, the median pack-year history was 5 (IQR: 2.4–24). A total of 15 participants (6.9%) reported chewing chat, and 13 (6%) consumed alcohol.
A family history of early CVD was noted in only 3 participants (1.4%). Regular physical activity, defined as moderate exercise for at least 150 minutes per week, was reported by 50 participants (23.1%). Retroviral infection was identified in 14 participants (6.5%), all of whom were on antiretroviral therapy. Among female participants, 11 (16.4%) were postmenopausal.
The BMI was 24.7 kg/m² (95% CI: 20.2–29.2). The average waist and hip circumferences were 89.8 cm (95% CI: 74.4–104.8) and 95.2 cm (95% CI: 80.9–109.2), respectively, with a mean waist-to-hip ratio of 0.9 (95% CI: 0.8–1.0). The average ABI was 1.1 (95% CI: 0.8–1.4) on the right and 1.2 (95% CI: 0.9–1.5) on the left.
|
Table 3. Physical measures and behavioral risk factors of cardiovascular disease in CKD patients at TASH renal clinic, Addis Ababa, Ethiopia, 2021 |
||
|
Variables |
Frequency (n=216) |
Percentage (%) |
|
Smoking status |
|
|
|
Current smoker |
6 |
2.8 |
|
Ex-smoker |
22 |
10.2 |
|
Never smoker |
188 |
87.0 |
|
First degree relative with premature cardiovascular disease |
3 |
1.4 |
|
Active chewing chat |
15 |
6.9 |
|
Active alcohol drinking |
13 |
6.0 |
|
Physical exercise at least 30 minutes 3*/week |
50 |
23.1 |
|
Is the patient at menopause (for females n= 67) |
11 |
16.7 |
|
Serostatus for HIV |
|
|
|
Positive |
14 |
6.5 |
|
Negative |
180 |
83.3 |
|
Unknown |
22 |
10.2 |
|
BMI (n=75) |
|
|
|
<18.5 kg/m2 |
3 |
4.0 |
|
18.5-24.9 kg/m2 |
42 |
56.0 |
|
BMI – body mass index, CKD – chronic kidney disease, HIV – human immunodeficiency virus, TASH -Tikur Anbessa Specialized Hospital |
||
Cardiovascular disease characteristics
Among the study participants, 31 (14.4%) had IHD, 24 (11.1%) had cerebrovascular disease, and 9 (4.2%) had PAD. The overall prevalence of ASCVD, encompassing IHD, cerebrovascular events, and PAD, was 27.8% (95% CI: 21.9%–34.3%). Of those with cerebrovascular disease, 13 (54.2%) had ischemic stroke, 10 (41.7%) had hemorrhagic stroke, and 1 patient had a documented TIA.
Among patients with IHD, only 3 (9.7%) had undergone coronary angiography and all had received percutaneous coronary intervention. The remaining cases were managed medically. Notably, all patients with ASCVD were on aspirin, and 57 (95%) were receiving statin therapy at the time of the study.
Echocardiographic assessments revealed LVH in 66 participants (30.6%). Systolic dysfunction was identified in 46 participants (21.3%), while diastolic dysfunction was present in 61 participants (28.2%) (Table 4). Of those with systolic dysfunction, 33 (71.7%) had mild, 10 (21.7%) moderate, and 3 (6.5%) severe left ventricular dysfunction. Among those with diastolic dysfunction, 52 (78.8%) had grade I, 10 (15.2%) grade II, 3 (4.6%) grade III, and 1 (1.5%) grade IV diastolic dysfunction. The mean left ventricular ejection fraction was 57.6% (95% CI: 46.3%–70.9%).
Only two participants in the study had cardiac prosthetic devices, both of which were permanent pacemakers.
|
Table 4. Cardiovascular disease characteristics of CKD patients at TASH renal clinic, Addis Ababa, Ethiopia, 2021 |
||
|
Variables |
Frequency (n=216) |
Percentage (%) |
|
Ischemic heart disease |
31 |
14.4 |
|
Cerebrovascular disease Total |
24 |
11.1 |
|
Ischemic stroke Hemorrhagic stroke |
13 10 |
6.0 4.6 |
|
TIA |
1 |
0.5 |
|
Peripheral arterial disease |
9 |
4.2 |
|
Echocardiography |
|
|
|
Normal echocardiography |
109 |
50.5 |
|
LVH |
61 |
28.2 |
|
IHD |
19 |
8.8 |
|
Diastolic dysfunction |
66 |
30.6 |
|
Systolic dysfunction |
46 |
21.3 |
|
Pulmonary hypertension |
11 |
5.1 |
|
CKD – chronic kidney disease, IHD – ischemic heart disease, LVH – left ventricular hypertension, TIA – transient ischemic attack TASH -Tikur Anbessa Specialized Hospital |
||
The correlation of independent variables and CVD
In this study, several independent variables demonstrated a statistically significant positive correlation with the presence of CVD. Age showed a weak but significant positive correlation with CVD, with a correlation coefficient of r (216) = 0.190 (p = 0.005). eGFR) was also positively correlated with CVD, r(216) = 0.898 (p = 0.009), as was total cholesterol, r(216) =
0.916 (p = 0.008). Additionally, smoking pack-years showed a moderate positive correlation with CVD, r(216) = 0.456 (p = 0.048). These findings highlight the significant associations between both traditional and CKD-related risk factors and CVD among pre-dialysis CKD patients. The details of these correlations are summarized in Table 5.
|
Table 5. The correlation independent variable and cardiovascular disease in CKD patients at TASH renal clinic Addis Ababa, Ethiopia, 2021 |
|||
|
Variables |
Pearson Correlation |
N |
p |
|
Age |
0.19 |
216 |
0.005 |
|
eGFR |
0.898 |
216 |
0.009 |
|
24 hours |
0.128 |
106 |
0.191 |
|
FBS |
-0.026 |
166 |
0.740 |
|
HbA1C |
-0.063 |
119 |
0.497 |
|
Total cholesterol |
0.916 |
162 |
0.008 |
|
Triglyceride |
0.046 |
163 |
0.564 |
|
Hemoglobin |
0.145 |
216 |
0.034 |
|
Hematocrit |
0.197 |
199 |
0.005 |
|
Calcium |
-0.048 |
147 |
0.562 |
|
Smoking pack year |
0.459 |
190 |
0.048 |
|
CKD – chronic kidney disease, eGFR – estimated glomerular filtration rate, FBS – fasting blood glucose, hemoglobin A1C, TASH -Tikur Anbessa Specialized Hospital |
|||
Determinants of cardiovascular disease
The strength of association between independent variables and CVD was evaluated using both bivariate and multivariable logistic regression analyses (Table 6). In the bivariate analysis, age, sex, and office blood pressure were significantly associated with CVD. In the multivariable logistic regression model, an estimated glomerular filtration rate (eGFR) of 30–45 mL/min/1.73 m² was found to be protective, with participants in this group being 69% less likely to develop cardiovascular CVD; chat was associated with a markedly increased risk, with chat chewers having 5.1 times higher odds of developing CVD compared to non-chewers (AOR = 5.1, 95% CI: 1.10–24.66). Similarly, participants with uncontrolled blood pressure (≥140/90 mmHg) were 4.7 times more likely to have CVD compared to those with lower blood pressure readings (AOR = 4.7, 95% CI: 1.17–18.71).
|
Table 6. The association of risk factors and cardiovascular disease using binary logistic regression among participant having CKD in Addis Ababa, Ethiopia, 2021 |
||||
|
Variables |
Bivariate COR (95% CI) |
p |
Multivariate AOR (95% CI) |
p |
|
Gender Male Female |
2.2(1.1-3.3) |
0.0321 |
1.6(0.46, 5.55) |
0.467 1 |
|
Age category ≤29 years 30-39 years 40-49 years 50-59 years ≥60 years |
0.17(0.02, 1.37) 0.25(0.07, 0.91) 0.66(0.28, 1.57) 0.39(0.17, 0.89) |
0.096 0.035 0.347 0.0251 |
0.65(0.05, 8.85) 0.12(0.01, 1.26) 0.24(0.04, 1.45) 0.29(0.08, 5.47)
|
0.744 0.077 0.120 0.063 1 |
|
eGFR, ml/min/1.72m2 <30 30-45 >45 |
0.67(0.31, 1.45) 1.5(0.74, 3.1) |
0.312 0.2591 |
0.71(0.24, 5.12) 0.31(0.09, 0.45) |
0.536 0.040 1 |
|
DM |
1.5(0.9, 2.1) |
0.212 |
1.253(0.667-2.36) |
0.484 |
|
FBS >130 mg/dL |
1.3(0.66, 2.54) |
0.457 |
1.4(0.52, 3.55) |
0.53 |
|
Office BP ≥140/90 mmHg |
2.9(1.16, 7.58) |
0.023 |
4.7(1.17, 18.71) |
0.029 |
|
Chat chewing |
1.8(0.62, 5.34) |
0.279 |
5.1(1.1, 24.66) |
0.042 |
|
BP – blood pressure, CKD – chronic kidney disease, DM – diabetes mellitus, eGFR – estimated glomerular filtration rate, FBS – fasting blood glucose, hemoglobin A1C, TASH -Tikur Anbessa Specialized Hospital |
||||
Discussion
This study demonstrates a substantial prevalence of ASCVD, notably ischemic heart disease and cerebrovascular disease, among pre-dialysis CKD patients. It further emphasizes the significant burden of both traditional and non-traditional CV risk factors contributing to the heightened cardiovascular morbidity observed in this population. CVD is a well-established complication across all stages of CKD, contributing significantly to morbidity and mortality. Patients with CKD often exhibit a combination of traditional cardiovascular risk factors—such as hypertension, diabetes mellitus, and dyslipidemia—as well as CKD-specific, non-traditional factors including anemia, chronic inflammation, and mineral-bone disorders. CKD is increasingly recognized as a major public health concern, particularly in low- and middle-income countries.
Although there are no population-based studies on CKD prevalence in Ethiopia, a systematic review in Sub-Saharan Africa estimated a regional prevalence of approximately 14%. In Ethiopia, CKD has been reported in 35.5% of diabetic patients and 22.1% of those with hypertension, underscoring the need for early detection and integrated CV risk management in these high-risk groups (2,19–21).
In our cohort, more than two-thirds of participants were male, aligning with findings from similar studies. Diabetes mellitus emerged as the leading cause of CKD, consistent with global epidemiological patterns. This finding contrasts with earlier reports from Ethiopia, which identified hypertension and undiagnosed glomerulonephritis as the predominant etiologies. The observed shift may reflect increasing urbanization, lifestyle changes, and the growing burden of metabolic syndrome. Furthermore, the relatively older age of our study population may have contributed to the higher prevalence of DM. The distribution of CKD stages was relatively balanced, with approximately one-third of participants in each of stages 3a, 3b, and 4 (20,22).
Hypertension was present in over 91% of participants—markedly higher than the 15.7% prevalence reported in the general Ethiopian population. This observation is consistent with findings from previous studies, including Babua et al. (13), which reported a 90% prevalence of hypertension among patients with CKD. In our cohort, one-third of participants were presumed to have hypertension as the underlying etiology of CKD. However, establishing causality remains challenging in the absence of renal biopsy or longitudinal data. Notably, the majority of patients had controlled blood pressure, with only 10% recording office blood pressure values ≥140/90 mmHg. Although the optimal target for blood pressure control in CKD continues to be a subject of ongoing debate, current international guidelines recommend maintaining systolic blood pressure between 130 and 139 mmHg. Despite this, only two-thirds of participants were receiving renin-angiotensin-aldosterone system inhibitors, indicating suboptimal adherence to guideline-recommended therapy (13,23,24).
The prevalence of DM in our cohort was notably high at 48.1%, significantly exceeding the national estimate of 3.2% and surpassing rates reported in comparable CKD studies. This elevated prevalence may be attributed to diabetes mellitus serving both as a major risk factor and as a leading cause of CKD. Despite the well-established renal and cardiovascular benefits of SGLT2 inhibitors, only a small proportion of patients in our study were receiving these agents. Their underutilization is likely due to limited access and affordability in the local healthcare setting (25, 26).
The mean hemoglobin level in our cohort was 13.4 g/dL, which is slightly higher than that reported in comparable studies—likely reflecting the earlier CKD stages of our participants. Despite this, 30% of individuals had hemoglobin levels below 12 g/dL, and only a small proportion were receiving treatment for anemia, underscoring a significant gap in care. In contrast, a study from Uganda documented hemoglobin levels below 11 g/dL in 71.9% of CKD patients; however, their cohort included a higher proportion of individuals with stage 5 CKD, which may account for the greater severity of anemia observed(13, 27).
The mean LDL cholesterol level in our cohort was 105 mg/dL, comparable to global CKD cohorts and lower than other high-risk populations in Ethiopia, likely due to the high rate of statin use (55.6%). Hypercholesterolemia prevalence (18.5%) was higher than the national average (5.2%), as expected in this high-risk group (25).
The prevalence of HIV in our cohort was 6.5%, markedly higher than the national average of 0.9%, yet lower than that reported in comparable regional studies, including one from Uganda. HIV remains a significant risk factor for the development of CKD in Sub-Saharan Africa. With respect to nutritional status, 40% of participants were classified as overweight, while none met the criteria for obesity. Notably, in advanced stages of CKD, a lower body mass index (BMI) has been paradoxically associated with poorer clinical outcomes—a phenomenon referred to as reverse epidemiology (28–30).
The ASCVD in our cohort was 27.8%, aligning with findings from several international CKD cohorts, including MERENA (Spain, 39.1%), CRISIS (UK, 47.2%), CRIC (US, 33.4%), CKD-ROUTE (Japan, 26.8%), and C-STRIDE (China, 9.8%). The relatively lower prevalence of PAD observed in our study may be attributable to underdiagnosis due to limited diagnostic resources.
When compared to the estimated 5% ASCVD prevalence in the general Ethiopian population, the markedly higher rate observed among CKD patients underscores the critical need for early CV risk assessment and integrated intervention strategies within CKD management (7–11).
LVH was identified in nearly one-third of our participants, a prevalence that appears lower than expected given the high rate of hypertension in the cohort.
In comparison, Babua et al. (13) reported an LVH prevalence of 54.4% among CKD patients, likely reflecting more advanced disease stages and suboptimal blood pressure control in their study population. The prevalence of systolic dysfunction, defined as an ejection fraction <50%, was 21.3% in our cohort—slightly higher than the 18.9% reported in a Ugandan study. This difference may be attributable to a greater burden of IHD in our study population(13, 31).
In our study, age, eGFR, total cholesterol, and smoking pack-years were all positively correlated with the presence of ASCVD. While the associations with age, elevated cholesterol, and tobacco exposure are well established in the literature, the positive correlation with eGFR was unexpected. A possible explanation is that individuals with more advanced CKD and lower eGFR may be more likely to develop non-atherosclerotic CV conditions, such as uremic cardiomyopathy, which were not specifically assessed in this study. Alternatively, patients with lower eGFR may receive more intensive management of modifiable risk factors, thereby attenuating their ASCVD burden. Multivariable logistic regression analysis identified chat chewing and uncontrolled office blood pressure (≥140/90 mmHg) as independent predictors of ASCVD, with adjusted odds ratios of 5.1 and 4.7, respectively. Previous studies have associated chat chewing with increased risks of myocardial infarction and stroke. Likewise, uncontrolled hypertension remains a well-recognized contributor to CV morbidity in both the general and CKD populations(32–35).
Strengths of the study
A major strength of this study is that it is the first to assess ASCVD and associated risk factors among pre-dialysis CKD patients in Ethiopia, using a robust sample size and detailed clinical and echocardiographic data.
Study limitations
However, several limitations must be acknowledged. First, the single-center design may limit generalizability. Second, non-consecutive recruitment may introduce selection bias. Third, although the sample size was reasonable, it remains small relative to the broader CKD population.
Furthermore, most patients did not undergo renal biopsy, limiting the precision of CKD etiology classification. The diagnosis of IHD relied on clinical and echocardiographic criteria, with only three patients undergoing coronary angiography. Finally, the study focused on a limited set of CVDs and risk factors, which may underestimate the total CV burden in this population.
Conclusion
This study demonstrated a high prevalence of ASCVD among pre-dialysis CKD patients attending the renal clinic at Tikur Anbessa Specialized Hospital. In addition, there was a substantial burden of traditional and non-traditional cardiovascular risk factors, including diabetes mellitus, hypertension, anemia, and hypertriglyceridemia. These findings underscore the urgent need for early cardiovascular risk assessment and targeted interventions in this high-risk population.
Ethics: Data collection was carried out after approval of the research proposal by institutional review board of Department of Internal Medicine, School of Medicine, College of Health Sciences, Addis Ababa University. Appropriate measures were taken to protect confidentiality of the collected information. All collected data didn’t contain patient identifications like names, patient’s medical record number and residence addresses. Written informed consent was taken from eligible patients. Patients were provided with information on the objectives of the study and confidentiality issues. At the end of the session, patients were given the opportunity to enroll in the study at that time and continued the appropriate CKD care per Ethiopian Federal Ministry of Health and international standard of care guidelines. Those who want to discuss their participation in the study with family members were given an opportunity to do.
Peer-review- External and internal
Conflict of interest- None to declare
Authorship: Y.A.B., S.A.G., A.M.E., D.M., T.A.B., and S.G.A. equally contributed to the study
and fulfilled all authorship criteria for publication.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
References
| 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: A Statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42: 1050-65. https://doi.org/10.1161/01.HYP.0000102971.85504.7c PMid:14604997 |
||||
| 2.Go AS, Chertow GM, Fan D, Mcculloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296-305. https://doi.org/10.1056/NEJMoa041031 PMid:15385656 |
||||
| 3.Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimbürger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? J Am Soc Nephrol 2008; 3: 505-21. https://doi.org/10.2215/CJN.03670807 PMid:18184879 PMCid:PMC6631093 |
||||
| 4.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 2006; 17: 2034-47. https://doi.org/10.1681/ASN.2005101085 PMid:16738019 |
||||
| 5.Coresh J. Association of estimated glomerular fi ltration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073-81. https://doi.org/10.1016/S0140-6736(10)60674-5 PMid:20483451 |
||||
| 6. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. J Int Soc Nephrol 2012. Available at: URL: https://kdigo.org | ||||
| 7.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, et al. The Chronic Renal Insufficiency Cohort (CRIC) study. J Am Soc Nephrol 2003; 14 (suppl_2): S148-53. https://doi.org/10.1097/01.ASN.0000070149.78399.CE PMid:12819321 |
||||
| 8.Chadban SJ, Briganti EM, Kerr PG, Dunstan DW, Welborn TA, Zimmet PZ, et al. Prevalence of kidney damage in Australian adults: The AusDiab kidney study. J Am Soc Nephrol 2003; 14 (Suppl 2): S131-8. https://doi.org/10.1097/01.ASN.0000070152.11927.4A PMid:12819318 |
||||
| 9.Amenós AC, González-Juanatey JR, Gutiérrez PC, Gilarranz AM, Costa CG. Prevalence of chronic kidney disease in patients with or at a high risk of cardiovascular disease. Rev Españ Cardiol 2010; 63: 225-8. https://doi.org/10.1016/S0300-8932(10)70041-5 |
||||
| 10.Iimori S, Noda Y, Okado T, Naito S, Toda T, Chida Y, et al. Baseline characteristics and prevalence of cardiovascular disease in newly visiting or referred chronic kidney disease patients to nephrology centers in Japan: A prospective cohort study. BMC Nephrol 2013; 14: 152. https://doi.org/10.1186/1471-2369-14-152 PMid:23865418 PMCid:PMC3723419 |
||||
| 11.Yuan J, Zou XR, Han SP, Cheng H, Wang L, Wang JW, et al. Prevalence and risk factors for cardiovascular disease among chronic kidney disease patients: Results from the Chinese cohort study of chronic kidney disease (C-STRIDE). BMC Nephrol 2017; 18: 23. https://doi.org/10.1186/s12882-017-0441-9 PMid:28088175 PMCid:PMC5237491 |
||||
| 12.Oguntola SO, Hassan MO, Duarte R, Vachiat A, Manga P, Naicker S. Atherosclerotic vascular disease is more prevalent among black ESKD patients on long-term CAPD in South Africa. BMC Nephrol 2019; 20: 399. https://doi.org/10.1186/s12882-019-1583-8 PMid:31666030 PMCid:PMC6821013 |
||||
| 13.Babua C, Kalyesubula R, Okello E, Kakande B, Sebatta E, Mungoma M, et al. Pattern and presentation of cardiac diseases among patients with chronic kidney disease attending a national referral hospital in Uganda: A cross sectional study. BMC Nephrol 2015;16: 126. https://doi.org/10.1186/s12882-015-0128-z PMid:26238594 PMCid:PMC4522958 |
||||
| 14.Arodiwe EB, Arodiwe EB, Ulasi II, Ijoma CK, Ike SO. Left ventricular diastolic function in a predialysis patient. West African Journal of Medicine 2010; 29: 225-9.. https://doi.org/10.4314/wajm.v29i4.68231 PMid:20931508 |
||||
| 15.Chijioke A, Makusidi AM, Kolo PM. Electrocardiographic abnormalities among dialysis naïve chronic kidney disease patients in Ilorin Nigeria. Ann Afr Med 2012; 11: 21-6. https://doi.org/10.4103/1596-3519.91011 PMid:22199043 |
||||
| 16.Chillo P, Mujuni E. Prevalence and predictors of left ventricular dysfunction among patients with chronic kidney disease attending Muhimbili National Hospital in Tanzania: A cross-sectional study. Res Rep Clin Cardiol 2018; 9: 11-21. https://doi.org/10.2147/RRCC.S159472 |
||||
| 17.Major RW, Cheng MRI, Grant RA, Shantikumar S, Xu G, Oozeerally I, et al. Cardiovascular disease risk factors in chronic kidney disease: A systematic review and meta-analysis. PLoS One 2018; 13: e0192895 https://doi.org/10.1371/journal.pone.0192895 PMid:29561894 PMCid:PMC5862400 |
||||
| 18.Lu Z, Lu F, Zhang R, Guo S. Interaction between anemia and hyperuricemia in the risk of all-cause mortality in patients with chronic kidney disease. Front Endocrinol (Lausanne). 2024; 15: 1286206. https://doi.org/10.3389/fendo.2024.1286206 PMid:38586465 PMCid:PMC10998448 |
||||
| 19.Hunegnaw A, Mekonnen HS, Techane MA, Agegnehu CD. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients at Northwest Amhara Referral Hospitals, Northwest Ethiopia, 2020. Int J Hypertens 2021; 2021: 5515832. https://doi.org/10.1155/2021/5515832 PMid:34484816 PMCid:PMC8416396 |
||||
| 20.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: Global dimension and perspectives. Lancet 2013; 382: 260-72. https://doi.org/10.1016/S0140-6736(13)60687-X PMid:23727169 |
||||
| 21.Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health 2014; 2: e174-81. https://doi.org/10.1016/S2214-109X(14)70002-6 PMid:25102850 |
||||
| 22.Sertsu A, Worku T, Fekadu G, Tura AK. Prevalence of chronic kidney disease and associated factors among patients visiting renal unit of St. Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia: A cross-sectional study design. SAGE Open Med 2022; 10: 20503121221116942. https://doi.org/10.1177/20503121221116942 PMid:35966210 PMCid:PMC9373155 |
||||
| 23.Bahrey D, Gebremedhn G, Mariye T, Girmay A, Aberhe W, Hika A, et al. Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes 2019; 12: 562. https://doi.org/10.1186/s13104-019-4610-8 PMid:31500655 PMCid:PMC6734391 |
||||
| 24.Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for themanagement of arterial hypertension. Vol. 39, Eur Heart J 2018; 39: 3021-104. https://doi.org/10.1093/eurheartj/ehy339 PMid:30165516 |
||||
| 25.Ethiopia steps report on risk factors for non-communicable diseaes and prevalence of selected ncds. 2016. Available at: URL: www.issup.net | ||||
| 26.Abuhay HW, Yenit MK, Melese M, Alemu GG, Aragaw FM. Prevalence and associated factors of chronic kidney disease among diabetes mellitus patients in Ethiopia: A systematic review and meta-analysis. PLoS One 2025; 20: 0315529. https://doi.org/10.1371/journal.pone.0315529 https://doi.org/10.1371/journal.pone.0316160 PMid:39888910 PMCid:PMC11785277 |
||||
| 27.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, et al. Health-related quality of life and hemoglobin levels in chronic kidney disease patients. J Am Soc Nephrol 2009; 4: 33-8. https://doi.org/10.2215/CJN.00630208 PMid:18987300 PMCid:PMC2615698 |
||||
| 28.Ethiopia Demographic and Health Survey. 2016. Available: at: URL: www.dhsprogramme.com | ||||
| 29.Ssemasaazi AJ, Kalyesubula R, Manabe YC, Mbabazi P, Naikooba S, Ssekindi F, et al. Higher prevalence of kidney function impairment among older people living with HIV in Uganda. BMC Nephrol 2024; 25: 321. https://doi.org/10.1186/s12882-024-03761-1 PMid:39334034 PMCid:PMC11428404 |
||||
| 30.Kalantar-Zadeh K. Energy balance and body composition in chronic uremia: causes and consequences of the reverse epidemiology of body mass index in dialysis patients.XXXXXX 2005; XX: XX https://doi.org/10.1053/j.jrn.2004.09.020 PMid:15648024 |
||||
| 31.Taddei S, Nami R, Bruno RM, Quatrini I, Nuti R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail Rev 2011; 16: 615-20. https://doi.org/10.1007/s10741-010-9197-z PMid:21116711 |
||||
| 32.Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int 2015; 88: 622-32. https://doi.org/10.1038/ki.2015.142 PMid:25945406 PMCid:PMC4556588 |
||||
| 33.Ku E, Lee BJ, Wei J, Weir MR. Hypertension in CKD: Core Curriculum 2019. Am J Kidney Dis 2019; 74: 120-31. https://doi.org/10.1053/j.ajkd.2018.12.044 PMid:30898362 |
||||
| 34.Chen F, Liu J, Han S, Xu T. Association between 10-Year Atherosclerotic Cardiovascular Disease Risk and Estimated Glomerular Filtration Rate in Chinese People with Normal to Slightly Reduced Kidney Function: A Cross-Sectional Study. Int J Environ Res Public Health 2022; 19: 6713. https://doi.org/10.3390/ijerph19116713 PMid:35682297 PMCid:PMC9180408 |
||||
| 35.Al-Motarreb A, Briancon S, Al-Jaber N, Al-Adhi B, Al-Jailani F, Salek MS, et al. Khat chewing is a risk factor for acute myocardial infarction: A case-control study. Br J Clin Pharmacol 2005; 59: 574-81. https://doi.org/10.1111/j.1365-2125.2005.02358.x PMid:15842556 PMCid:PMC1884851 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

