Multimodality cardiovascular imaging in the complete congenital absence of the pericardium: case report and brief literature review
CASE REPORT
Multimodality cardiovascular imaging in the complete congenital absence of the pericardium: case report and brief literature review
Article Summary
- DOI: 10.24969/hvt.2025.577
- Cardiac Surgery
- Published: 03/07/2025
- Received: 23/05/2025
- Revised: 20/06/2025
- Accepted: 21/06/2025
- Views: 2327
- Downloads: 1453
- Keywords: Congenital absence of the pericardium, echocardiography, computed tomography, cardiac magnetic resonance imaging
Address for Correspondence: Nelya Oryshchyn, Diagnostic Radiology Department, Danylo Halytsky Lviv National Medical University, Lviv Regional Centre of Cardiology, Lviv, Ukraine, 79015
Email: oryshchyn_n@yahoo.com
ORCID: Nelya Oryshchyn - 0000-0003-3758-9181; Yurij Ivaniv - 0000-0002-2153-9262; Veronika Yevtukh - 0000-0001-6737-7791; Anastasiya Oryshchyn - 0009-0005-4617-5054
Nelya Oryshchyn1,2, Yurij Ivaniv1, Veronika Yevtukh1, Anastasiya Oryshchyn2
1Diagnostic Radiology Department, Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
2 Cardiology Department, Lviv Regional Centre of Cardiology, Lviv, Ukraine
Abstract
Objective: Congenital absence of the pericardium (CAP) is a rare heart disorder, frequently misdiagnosed due to an unspecific clinical picture and leading to diagnostic challenges. The purpose of this case presentation is to show how cardiac imaging methods can aid in accurate diagnosis of CAP.
Case presentation: A case of the complete congenital absence of the pericardium in a 40-year-old man with complaints on dyspnea and fatigue is presented. Echocardiography revealed a dilated right ventricle with good contractility; normal dimensions and function of the left ventricle, and normal heart valve function. Pulmonary hypertension and atrial septal defect were excluded during echocardiography. Computed tomography revealed abnormal heart axis rotation leftward and posteriorly, raising suspicion of CAP. The diagnosis was confirmed by cardiac magnetic resonance imaging . The diagnostic flowchart for the CAP is discussed.
Conclusion: Multimodality cardiac imaging provides clues to the diagnosis of CAP.

Key words: Congenital absence of the pericardium, echocardiography, computed tomography, cardiac magnetic resonance imaging
Introduction
Congenital absence of the pericardium (CAP) is a rare disorder caused by an embryonal defect of the pericardium, which could be complete or partial (1, 2). CAP is an extremely rare pathology, as of 2023, there have been reported only 500 cases in the literature.
Without a pericardium, the heart lacks a fixation point in the chest, and its mobility is exaggerated, causing symptoms of fatigue, palpitations, and dyspnea. The position of the heart in the chest without pericardial fixation changes with rotation of the apex leftward and posteriorly, the right ventricle appears visually dilated.
CAP is a rare condition that has been reported in the literature until 2023 in only approximately 500 cases (1). Previously reported incidence of CAP was 1 in 10 000 (2).
Due to non-specific symptoms, CAP can mimic other heart diseases such as acute coronary syndrome, atrial septal defect, pulmonary embolism, etc. Non-specific symptoms, which could lead to misdiagnosis of coronary artery disease, are chest pain and dyspnea during physical exertion. It can lead to misinterpretation and unnecessary diagnostic procedures (coronary angiography). Dyspnea poses a question of differential diagnosis with pulmonary embolism, chronic pulmonary hypertension, atrial septal defect with chronic right heart volume overload.
Diagnosis of CAP is challenging because many patients with total CAP are asymptomatic and existing symptoms are non-specific, making it difficult to accurately determine the true incidence of the CAP. Therefore, awareness of this pathology is crucial for planning diagnostic workup. Because of potential complications such as heart strangulation, chest pain or even sudden cardiac death in patients with partial CAP, it is important to review this topic. Methods of cardiac imaging can aid in establishing the correct diagnosis. While echocardiography raises the suspicion of CAP, other methods, such as cardiac computed tomography (CT) and magnetic resonance imaging (MRI), confirm the diagnosis.
The aim of the study is to describe the radiological clues to the diagnosis of CAP, and give evidence of the role of multimodality imaging in the diagnosis of CAP based on our experience. A clinical case of a male patient with non-specific complaints and right ventricular dilatation revealed by echocardiography is presented. His medical history from the previous hospital listed several suspicious diagnoses, including pulmonary artery embolism, atrial septal defect, and patent foramen ovale. The purpose of the study is to share information about this rare pathology and to discuss specific changes obtained by cardiac imaging for this diagnosis. The methods of differentiation of causes of right ventricular (RV) dilatation in this case and the role of multimodality imaging in diagnosing CAP are presented.
Case report
Patient information: A 40-year-old male patient was admitted to the cardiological department with complaints of non-specific chest pain, dyspnea and fatigue on physical exertion. Throughout his life, he got used to physical training without participation in competitive sports. His physical activity increased significantly over the last time. He had complaints of dyspnea and fatigue during the last two months. Two weeks before index admission, he was hospitalized in a general hospital for shortness of breath, where diagnoses of atrial septal defect and pulmonary embolism were suspected. The patient was prescribed anticoagulation with rivaroxaban 20 mg (for suspected pulmonary embolism) and carvedilol 6,25 mg for mild arterial hypertension. The patient had no significant past medical history, including no history of cardiovascular disease, cerebrovascular disease, or substance use (tobacco or alcohol). The patient denied any family history of cardiovascular disorders.
Clinical findings: During physical examination, heart sounds were regular, with a heart rate of 80-90 per minute, and no heart murmur was revealed. Lateral displacement of the maximal heart sounds auscultation point was found. Blood pressure was 140/85 mm Hg. No signs of peripheral congestion were revealed.
Diagnostic assessment: Laboratory work-up was non-remarkable. Electrocardiography (ECG) showed sinus rhythm and left heart axis deviation. Holter 48-hour ECG monitoring was unremarkable for major atrial or ventricular arrhythmic events. The ECG exercise stress test was unremarkable.
Imaging: The diagnostic procedure began with a chest X-ray and echocardiography. Finally, we performed cardiac CT and cardiac MRI to confirm the diagnosis.
On chest X-ray, the right heart border was absent. This finding was analyzed carefully later after echocardiographic assessment and raised the suspicion of leftward heart rotation, characteristic of congenital absence of the pericardium.
Echocardiography revealed RV dilatation (end-diastolic diameter 4 cm) in parasternal long-axis view (Fig.1) with good (rather hyperkinetic) RV systolic function, tricuspid annular plane systolic excursion (TAPSE) 25 mm. The left ventricle was not dilated (end-diastolic diameter 52 mm) with normal systolic function (ejection fraction 65%).
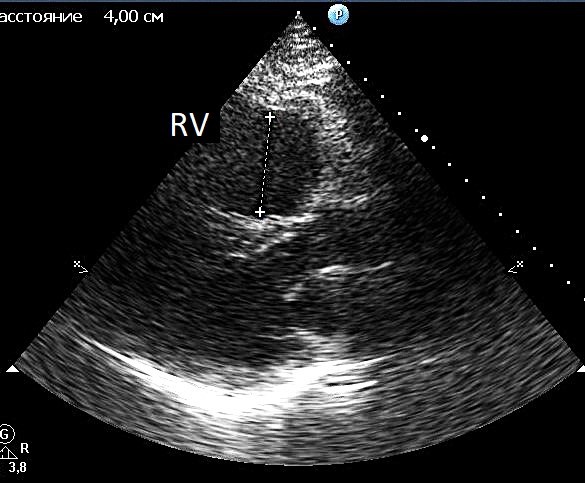
Figure. 1. Echocardiography. Right ventricular dilatation in parasternal long-axis view
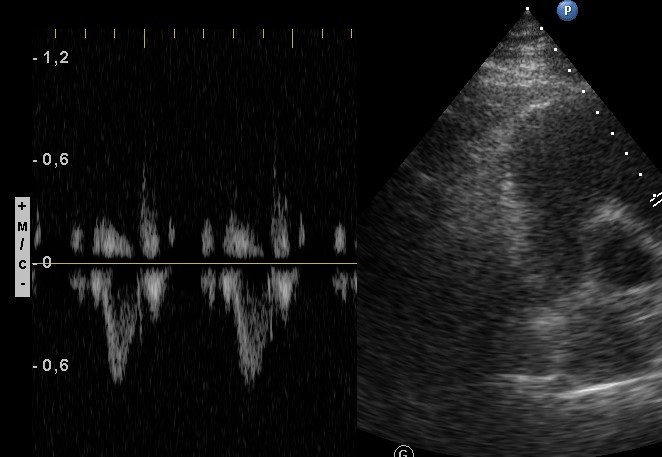
Fig. 2. Echocardiography. Normal pulmonary artery flow pattern in Doppler-echocardiography
No signs of pulmonary hypertension were revealed (no D-shaped left ventricle, no flattening of the interventricular septum, and no changes in the pulmonary artery flow pattern in Doppler-echocardiography) (Fig. 2).
Attempts to obtain an apical 4-chamber view from a conventional apical window were unsuccessful. This view was obtained from the axillary region, and the resolution was better with the patient sitting. The heart ventricles appeared to be wide in comparison with the small atria (Fig. 3, a). Small atrial dimensions and wide ventricles formed a teardrop-like shape of the heart (Fig.3, b). The atrial septal defect was excluded, and the flow in the pulmonary artery was not increased. We thoroughly examined the tricuspid valve in the RV inflow view and did not reveal any tricuspid regurgitation, which could result in the RV dilatation.
Considering the absence of pulmonary hypertension, atrial septal defect, significant tricuspid regurgitation and the impossibility of obtaining a cardiac image from a conventional apical view, the rare cause of RV dilatation – complete CAP was suspected. Absence of the right heart border on X-ray strengthened this suspicion.
A multidisciplinary team, which consisted of a cardiologist, radiologist and cardiac surgeon, decided that further evaluation with cardiac CT and MRI was needed.
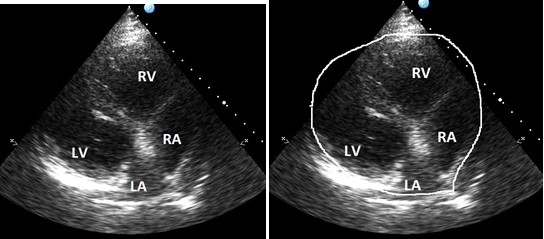
Figure 3. Echocardiography. Four –chamber apical view obtained from axillary region. Widened heart ventricles and small atrial dimensions. A tear-drop shape of the heart (b).
Cardiac computed tomography (CT) with contrast and cardiac magnetic resonance imaging (MRI) were performed. Both cardiac CT and MRI showed an extreme leftward rotation of the heart (Fig. 4, 5).
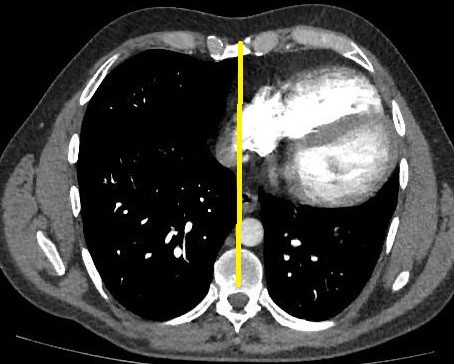
Figure 4. Cardiac CT. Extreme leftward and posterior rotation of the heart
CT – computed tomography
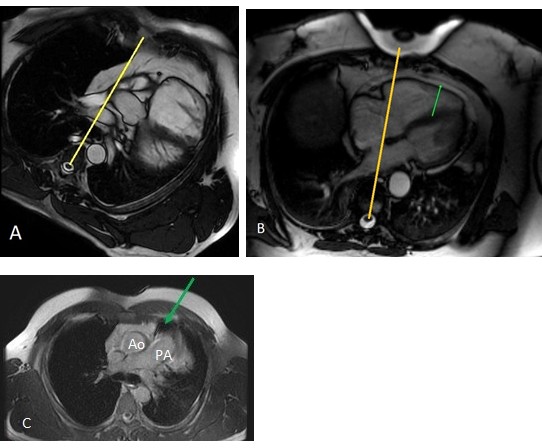
Figure 5. Absence of the pericardial layer in patients’ cardiac MRI (a) in contrast to normal heart position and normal finding of pericardial layer; (b) pericardium (green arrow); (c) axial MRI image demonstrates іnterposed lung tissue (green arrow) between the main pulmonary artery and the ascending aorta
MRI – magnetic resonance imaging
Сardiac CT did not reveal thrombi in pulmonary artery branches, including segmental and subsegmental branches. It allowed to exclude the diagnosis of pulmonary embolism. Leftward heart rotation confirmed the diagnosis of CAP (as an indirect sign), and pericardial layers were not detected on cardiac CT (Fig. 4). The decision was made to perform cardiac MRI. Cardiac MRI was performed with gadolinium to exclude the RV myocardial pathology.
Cardiac MRI confirmed extreme leftward and posterior rotation of the heart (Fig.5, a). It showed the absence of the pericardial layer in the patient (salient when compared with normal heart, Fig.5, b). Axial MRI image demonstrated іnterposed lung tissue (green arrow) between the main pulmonary artery and the ascending aorta (Fig.5, c). No late gadolinium enhancement was revealed.
Based on cardiac imaging results diagnosis of complete congenital absence of the pericardium was established by the multidisciplinary team members: cardiologist, cardiac surgeon, radiologist (specialist in cardiovascular imaging).
The treatment options were also discussed by the multidisciplinary team members.
Treatment: Taking into consideration the absence of significant hemodynamic changes, a beta-blocker (carvedilol 6.25 mg) was prescribed for the treatment for patient’s mild arterial hypertension and sinus tachycardia. Avoidance of excessive physical activity was recommended.
Follow-up and outcomes: Two years after the diagnosis of CAP was established, the patient was in stable clinical status. He decreased his physical activity, and he had not complaints during follow-up.
Discussion
The normal pericardium consists of two layers with a small amount of pericardial fluid among which acts as a lubricant during the cardiac cycle. Normal pericardium with its ligamentous attachment provides fixation of the heart in the chest (3, 4). CAP is a rare condition which results in the absence of normal tethering of heart chambers in the chest (1, 2, 3). The cushioning function of the pericardial sac is decreased. CAP can be divided into complete (as in the presented case) and partial form (left-sided or right-sided). In the complete CAP, the heart fixation in the thorax is lost, and the large vessels are the only sites of heart anchoring. As a result, the heart axis changes its position with extreme leftward and posterior orientation (5). The right ventricle, therefore, occupies a larger portion of the echocardiographic parasternal view anteriorly, as it is located anteriorly just after the chest wall. Movement of the heart becomes exaggerated, atrial dimensions are small, and the heart ventricles widen.
The total absence of pericardium could be asymptomatic or could cause non-specific symptoms (chest pain, dyspnea, fatigue) (6-8). The complete CAP is not likely to cause complications. The patients frequently have non-specific chest pain due to torsion of the great arteries (7-9). Cardiac arrhythmias were reported rarely. Partial CAP can be complicated by strangulation of cardiac structures through the defect of the pericardium, like herniation of the left atrial appendage or compression of coronary arteries (10-13). Sudden cardiac death was reported in a 12-year-old boy with a left-sided partial defect of the pericardium (10). Strangulations of the left ventricle or left atrial appendage were reported (11). Our patient had non-specific complaints of palpitation, and shortness of breath, mostly related to his increased physical exertion in the last month.
Chest X-ray could help to make a diagnosis: it shows an absence of the right heart border due to leftward heart rotation. Chest X-ray shows the interposition of pulmonary tissue between the aorta and pulmonary artery, and between the inferior heart wall and diaphragm (5, 14). In our case, the absence of the right ventricular border was evident, similar to reported cases.
The first finding usually obtained by echocardiography in CAP is the right ventricular RV dilation (15). In echocardiographic parasternal long-axis view, RV seems to be dilated: due to rotation of the heart, parasternal view shows the wider portion of the RV. RV dilatation, similar to our case, was reported frequently in CAP (10- 17). In our patient atrial septal defect was diagnosed previously due to RV dilatation, similar to that reported by Garnier et al (16). Rarely, right ventricular dilatation with RV systolic dysfunction in CAP is reported (17). It poses the need for differentiation from arrhythmogenic right ventricular cardiomyopathy. However, in our case, RV systolic function was preserved, excluding this diagnosis.
Right ventricular dilatation, revealed during echocardiography, requires differentiation between RV pressure or volume overload, RV myocardial pathology and rare causes (congenital absence of pericardium) (Table 1). Pulmonary artery stenosis and pulmonary hypertension are causes of RV pressure overload. Congenital septal defects and severe tricuspid regurgitation can lead to RV volume overload.
|
Table 1. Differential diagnostics of right ventricular dilatation (echocardiography) |
|
|
Disease |
Echocardiographic findings |
|
Pulmonary artery stenosis |
Turbulent high velocity flow and systolic pressure gradient on the pulmonary artery valve. Right ventricular wall hypertrophy. |
|
Pulmonary embolism |
D-shaped left ventricle, flattening of the interventricular septum, shortened acceleration time of the pulmonary artery flow. |
|
Chronic pulmonary hypertension |
D-shaped left ventricle, flattening of the interventricular septum, systolic pressure gradient on the tricuspid valve. Right ventricular wall hypertrophy. Dilatation of the right atrium and pulmonary artery |
|
Atrial septal defect |
Dilatation of both the right ventricle and the right atrium, increased flow velocity through the pulmonary artery valve. Direct visualization of atrial septal defect |
|
Arrhythmogenic right ventricular cardiomyopathy |
Right ventricular regional wall motion abnormalities. Right ventricular systolic function diminished |
|
Right ventricular myocardial infarction |
Right ventricular regional wall motion disturbances Right ventricular systolic function diminished. Inferior left ventricular myocardial infarction usually coexistent. |
|
Congenital absence of pericardium (total or left total) |
Normal right ventricular systolic function Normal right ventricular (and pulmonary artery) systolic pressure. Non-dilated right atrium and pulmonary artery. Impossibility to obtain apical view from the conventional apical echocardiographic window. |
Echocardiography could reveal or exclude signs of RV pressure or volume overload. RV myocardial pathology (arrhythmogenic cardiomyopathy, myocardial infarction, dilated cardiomyopathy) in contrast to CAP leads to diminished RV systolic function. The less known pathology is the congenital absence of pericardium
There are some echocardiographic clues to the diagnosis – it is impossible to obtain an apical view in the conventional approach, caused by leftward and posterior heart rotation, and small dimensions of heart atria. The diagnosis of the CAP should be confirmed by cardiac CT or MR.
Imaging in congenital absence of pericardium
Imaging findings rely mostly on the abnormal heart position and orientation of the heart in the chest. The findings are more specific for the total absence of pericardium, but in partial absence, they are more subtle due to the size and localization of the pericardial defect.
In patients with total absence of pericardium chest X-ray shows the leftward shift of the heart silhouette, loss of the right heart border (hidden by the spine), the interposition of the lung tissue between the aorta and pulmonary artery, and between the diaphragm and the base of the heart. In partial absence of the pericardium chest X-ray usually doesn’t reveal any changes until complications occur.
Echocardiography provides some specific clues to the diagnosis of congenital absence of pericardium. In patients with complete or total left absence of pericardium in parasternal view due to the leftward rotation of the heart wider portion of the right ventricle is seen, leading to the impression of a dilated right ventricle (18). The conventional apical window doesn’t provide a good apical view of the heart, because of the unusual position of the heart. Thus left lateral window from the axillary zone can be used, and the sitting position provides the best resolution (18, 19). The visualization of the apical view in our case was obtained from the axillary zone in the sitting position of the patient. It can serve as a tip for specialists in echocardiography in cases suspicious of CAP. In some reports of CAP, they considered echocardiography to be non-diagnostic (20), while it could provide some valuable information when a specific approach like an axillary window is used. The heart has a teardrop shape with the bulbous left ventricle, and the atria are elongated due to the absence of the tethering provided by the pericardium. Left ventricular systolic function is preserved.
Compared to echocardiography cardiac CT and CMR have a better sensitivity and specificity in identifying the abnormal position of the heart with its extreme leftward and posterior rotation and could demonstrate it more evidently.
Cardiac CT and MRI confirm the diagnosis of congenital absence of pericardium. In most cases presented, the diagnosis of CAP was confirmed by cardiac CT by cardiac leftward displacement and absence of pericardial layers (6, 7, 8, 14, 20).
The interposition of lung tissue between the aorta and pulmonary artery is also better seen in CT and MRI than in chest X ray (21). Elongation and straightening of the left heart border also have been reported (21, 22).
Cardiac MRI has been used as a diagnostic instrument to confirm the diagnosis with increasing frequency (8, 15, 17- 19). In our case, cardiac CT did not reveal a pericardial layer, but cardiac MRI was superior to CT in quality of visualization in demonstrating of the absence of pericardial layers.
Cardiac MRI shows pericardium like a line with medium to low signal in T1 and T2-weighted images, covered by the epicardial and mediastinal adipose tissue with high signal (Fig. 5, b). The presence of the epicardial fat with high signals makes visualization of the pericardium obtainable. Thus, the adipose tissue allows the visualization of the pericardium. However, in young person amount of adipose tissue along the pericardium might be low, so it could be complicated to reveal the pericardium. But the diagnosis of the CAP relies mostly on other signs – in patients with a total absence of pericardium leftward rotation of the heart is seen on axial images with posterolateral positioning of apex, which is seen both in cardiac CT and MRI (15, 19) (Table 2). The shape of the heart is teardrop-like with wide heart ventricles and small atria. Cardiac CT and MRI can distinguish between complete and partial defects of the pericardium, providing information about the possible risk of strangulation of part of heart chambers in partial forms (19). Our case demonstrates the role of cardiac MRI when the cause of RV dilation, revealed by echocardiography, is not completely understood.
|
Table 2. Direct and indirect signs to suggest CAP by cardiac CT and MRI |
|
|
Direct signs |
Indirect signs |
|
Absence or partial absence of the pericardium
|
A marked displacement of the heart into the left hemithorax in total and left CAP, the posterior orientation of the left ventricular apex |
|
The interposition of lung tissue between the aorta and main pulmonary artery and the main pulmonary artery and left atrial appendage |
|
|
The interposition of the lung tissue between the base of the heart and the diaphragm |
|
|
The "vertical cardiac flop" (scanning the patient in prone position) |
|
|
The herniation of chambers through a partial defect (a prominent convexity along the cardiac silhouette). |
|
|
CAP – complete absence of pericardium, CT – computed tomography, MRI – magnetic resonance imaging |
|
There is no clear evidence of the superiority of one diagnostic imaging method over another (cardiac CT versus cardiac MRI) in CAP. Cardiac CT has a greater spatial resolution and shorter acquisition time, but the ionising radiation exposure may limit its application. Both cardiac MRI and CT provide delineation of the pericardium as a curvilinear line utilizing pericardial fat as a contrast. CMR is helpful to exclude RV myocardial pathology, and CT is superior in excluding pulmonary embolism. CMR has a superiority in detecting partial CAP with herniation of heart chambers.
In waste majority of patients with complete CAP, no surgical intervention is needed, in contrast to partial absence, where surgery is needed in cases with a risk of heart chamber strangulation. However, extremely rarely, reconstruction of the pericardium in highly symptomatic patients is performed (in left-sided CAP) (22).
Multidisciplinary team decision is needed in patients with suspicion of CAP. The team should consist of cardiologist, radiologist (specialist in cardiac imaging), cardiac surgeon (especially in cases with partial CAP with increased risk of heart chambers herniation). Multiparametric cardiac imaging, including echocardiography, cardiac CT and MRI, should be applied and the results of imaging should be discussed.
Conclusions: Complete congenital absence of the pericardium is a rare pathology characterized by specific changes in cardiac imaging. Awareness of this pathology is needed to make the correct diagnosis and to avoid unnecessary medications.
Echocardiography reveals a dilated right ventricle in CAP, and there is a need for differentiation from RV myocardial pathology, volume or pressure overload. Radiological imaging by cardiac CT and MRI is necessary to confirm the diagnosis of CAP with direct signs (absence of the pericardial layer) or indirect signs (leftward and posterior heart rotation, the interposition of lung tissue between the aorta and main pulmonary artery and the main pulmonary artery and left atrial appendage). Our case underlines the role of multimodality imaging in the diagnosis of CAP and in defining the prognosis of the patient.
Take-home message
Right ventricular dilatation revealed by echocardiography in parasternal view without evidence of RV volume or pressure overload or myocardial dysfunction raises the suspicion of CAP. Apical echocardiographic view in patients with CAP is obtainable only from the axillary zone due to rotation of the heart. Cardiac computed tomography and magnetic resonance imaging ensure correct diagnosis, confirming leftward and posterior heart rotation in CAP and the absence of a pericardial layer.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Ethics: All procedures involving human participants were performed in accordance with the ethical standards of the institutional research committee. The patient provided consent for the publication of this case report and accompanying images
Peer-review: External and internal
Conflict-of-interest: The authors declare that they have no conflicts of interest.
Author contributions: O.N. designed the study research, analyzed the data, and wrote the manuscript. Y.V. and O.A. collected data and performed the research. I.Y. helped to review and edit the manuscript. All authors read, revised and approved the final manuscript. Thus, all authors fulfilled authorship criteria.
Acknowledgement and Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Contact authors. Sharing of raw material data should be in frame of fair use with acknowledgement of source and authors, or collaboration
References
| 1.Bassareo PP, Secinaro A, Ciliberti, P., et al. Congenital absence of pericardium: the largest systematic review in the field on 247 worldwide cases (1977-now). Cong Heart Dis 2023; 18: 595-610. 10.32604/chd.2023.046229. https://doi.org/10.32604/chd.2023.046229 |
||||
| 2.Southworth H, Stevenson CS. Congenital defects of the pericardium. Arch Inter nMed 1938; 00: 223-40. https://doi.org/10.1001/archinte.1938.00180080065006 |
||||
| 3.Nasser WK. Congenital diseases of the pericardium. Cardiovasc Clin 1976; 7: 271-86. | ||||
| 4.Shah AB, Kronzon I. Congenital defects of the pericardium: a review. Eur Heart J Cardiovasc Imaging 2015; 16: 821-7. https://doi.org/10.1093/ehjci/jev119 PMid:26003149 |
||||
| 5.Marzullo R, Capestro A, Cosimo R, Fogante M, Aprile A, Balardi L, et al. Congenital Absence of pericardium: the swinging heart. J Imaging 2024; 10: 199. doi: 10.3390/jimaging10080199 https://doi.org/10.3390/jimaging10080199 PMid:39194988 PMCid:PMC11355882 |
||||
| 6.Nikam R, Rapp J, Kandula A, Puram S, Saul D. Congenital absence of pericardium. Ann Pediatr Cardiol 2020; 13: 373-4. doi: 10.4103/apc.APC_137_20 https://doi.org/10.4103/apc.APC_137_20 PMid:33311934 PMCid:PMC7727896 |
||||
| 7.Li XY, Jiang Y, Li HW, Liu YK, Bai J. Congenital absence of the left pericardium: a case report. BMC Cardiovasc Disord 2023; 23: 247. doi: 10.1186/s12872-023-03262-3 https://doi.org/10.1186/s12872-023-03262-3 PMid:37173633 PMCid:PMC10176923 |
||||
| 8.Poskaite P, Pölzl G, Hangler H, Mayr A. Congenital absence of a left-sided pericardium. Eur Heart J Case Rep 2021; 5: ytab423. doi: 10.1093/ehjcr/ytab423 https://doi.org/10.1093/ehjcr/ytab423 PMid:34870087 PMCid:PMC8637791 |
||||
| 9.Cho H, Kang EJ, Kim MS, Jeong S, Lee KN. Incidentally detected pericardial defect in a patient with pneumothorax as confirmed on video-assisted thoracoscopic surgery. Taehan Yongsang Uihakhoe Chi 2021; 82: 749-55. doi: 10.3348/jksr.2020.0057 https://doi.org/10.3348/jksr.2020.0057 PMid:36238774 PMCid:PMC9432441 |
||||
| 10.Buyuk UY, Pakis I, Dogru A, Calk AU, Sudden death due to congenital pericardial defect: an autopsy case. Am J Forens Med Pathol 2008; 29: 242-4. https://doi.org/10.1097/PAF.0b013e318183f7de PMid:18725780 |
||||
| 11.Juárez AL, Akerström F, Alguacil AM, González BS. Congenital partial absence of the pericardium in a young man with atypical chest pain. World J Cardiol 2013; 5: 12-4. doi: 10.4330/wjc.v5.i2.12 https://doi.org/10.4330/wjc.v5.i2.12 PMid:23538774 PMCid:PMC3609010 |
||||
| 12.Wilson SR, Kronzon I, Machnicki SC, Ruiz CE. A constrained heart: a case of sudden onset unrelenting chest pain. Circulation 2014; 130: 1625-31. doi: 10.1161/CIRCULATIONAHA.114.011410 https://doi.org/10.1161/CIRCULATIONAHA.114.011410 PMid:25462822 |
||||
| 13.Izumoto S, Oguri N, Koyama S, Matsuura Y, Komatsu H, Asada Y, Iwakiri H. Cardiac strangulation due to partial pericardial defect presenting as acute myocardial infarction. JACC Case Rep 2021; 3: 1635-8. doi: 10.1016/j.jaccas.2021.07.029 https://doi.org/10.1016/j.jaccas.2021.07.029 PMid:34729518 PMCid:PMC8543160 |
||||
| 14.Aslan E, Maslak İC, Karabulut N. Congenital absence of the pericardium. Turk Kardiyol Dern Ars 2021; 49: 696-7. doi: 10.5543/tkda.2021.21132. PMID: 34881712 https://doi.org/10.5543/tkda.2021.21132 PMid:34881712 |
||||
| 15.Khayata M, Alkharabsheh S, Shah NP, Verma BR, Gentry JL, Summers M, et al. Case series, contemporary review and imaging guided diagnostic and management approach of congenital pericardial defects. Open Heart 2020; 7: e001103. doi: 10.1136/openhrt-2019-001103 https://doi.org/10.1136/openhrt-2019-001103 PMid:32076559 PMCid:PMC6999674 |
||||
| 16.Garnier F, Eicher JC, Philip JL, et al. Congenital complete absence of the left pericardium: a rare cause of chest pain or pseudo-right heart overload. Clin Cardiol 2010; 33: E52-7. doi: 10.1002/clc.20607 https://doi.org/10.1002/clc.20607 PMid:20043342 PMCid:PMC6653773 |
||||
| 17.Foo JS, Koh CH, Sahlén A, Tang HC, Lim CP. Congenital partial absence of pericardium: a mimic of arrhythmogenic right ventricular cardiomyopathy. Case Rep Med 2018; 2018: 4297280. doi: 10.1155/2018/4297280 https://doi.org/10.1155/2018/4297280 PMid:29849659 PMCid:PMC5914131 |
||||
| 18.Bernardinello V, Cipriani A, Perazzolo Marra M, Motta R, Barchitta A. Congenital pericardial agenesis in asymptomatic individuals: tips for the diagnosis. Circ Cardiovasc Imaging 2020; 13: e010169. doi: 10.1161/CIRCIMAGING.119.010169 https://doi.org/10.1161/CIRCIMAGING.119.010169 PMid:32370616 |
||||
| 19.Trimarchi G, Zito C, Pelaggi G, Carerj S, Di Bella G, Pericardial agenesis: a case report of a rare congenital heart disease, Eur Heart J Case Rep 2024; 8:, ytae200. doI: 10.1093/ehjcr/ytae200 https://doi.org/10.1093/ehjcr/ytae200 PMid:38690557 PMCid:PMC11060107 |
||||
| ami B, Alharbi A, Alhajji M, Gendi S, Hamirani YS. A case report of congenital absence of the pericardium that was diagnosed by cardiac computed tomography angiogram (CCTA). Radiol Case Rep 2022; 17:3380-4. doi: 10.1016/j.radcr.2022.06.066 https://doi.org/10.1016/j.radcr.2022.06.066 PMid:35874871 PMCid:PMC9304677 |
||||
| 21.Mekonnen S, Farris H, Azmeraw D. Complete Congenital absence of the left pericardium in elderly patient: a case report. Int Med Case Rep J 2024; 17: 347-352. doi: 10.2147/IMCRJ.S454910 https://doi.org/10.2147/IMCRJ.S454910 PMid:38646458 PMCid:PMC11032154 |
||||
| 22.Bouchard M, Hoschtitzky A, Gatzoulis M. Diagnosis and management of congenital absence of pericardium: a case report. Eur Heart J Case Rep 2019; 3: 1-5. doi: 10.1093/ehjcr/ytz223 https://doi.org/10.1093/ehjcr/ytz223 PMid:31911998 PMCid:PMC6939816 |
||||
| 23.Chetrit M, Xu B, Verma BR, Klein AL. Multimodality imaging for the assessment of pericardial diseases. Curr Cardiol Rep 2019;21: 41. doi: 10.1007/s11886-019-1115-y https://doi.org/10.1007/s11886-019-1115-y PMid:30993456 |
||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

