Differential diagnosis of aortic aneurysms: pathomorphological criteria of various etiological forms
ORIGINAL RESEARCH ARTICLE
Differential diagnosis of aortic aneurysms: pathomorphological criteria of various etiological forms
Article Summary
- DOI: 10.24969/hvt.2025.578
- CARDIOVASCULAR DISEASES
- Published: 09/07/2025
- Received: 05/06/2025
- Revised: 24/06/2025
- Accepted: 25/06/2025
- Views: 3185
- Downloads: 1239
- Keywords: Aorta, aortic aneurysm, Marfan syndrome, Takayasu syndrome, connective tissue, medial degeneration, pathomorphology
Address for Correspondence: Odilbek S. Sultanov, Republican Research Center Of Emergency Medicine, Tashkent,
Uzbekistan
E-mail: odilbek-2008@mail.ru
ORCID: Bokhodir A. Magrupov: 0000-0002-8795-0724; Abdusalom A. Abdurakhmanov: 0000-0003-0813-9333;
Khamidulla A. Abdumajidov: 0000-0001-5077-7174; Odilbek S. Sultanov: 0009-0005-7137-0185
Facebook: Bokhodir A. Magrupov - bokhodir.magrupov; Abdusalom A. Abdurakhmanov - abik.abikov; Khamidulla A.
Abdumajidov - khamidulla.abdumadjidov; Odilbek S. Sultanov- sultan.odil
Bokhodir A. Magrupov1,2, Abdusalom A. Abdurakhmanov2, Khamidulla A. Abdumajidov3, Odilbek S. Sultanov2
1Center for Development of Professional Qualifications of Medical Workers, Tashkent, Uzbekistan
2Republican Research Center of Emergency Medicine, Tashkent, Uzbekistan
3Bukhara State Medical Institute named after Abu Ali ibn Sina, Bukhara, Uzbekistan
Abstract
Objective: To identify morphological features of aortic wall structure in various pathologies that could serve as differential diagnostic criteria.
Methods: The study was conducted at the Pathology Department of the Republican Research Center for Emergency Medicine (Tashkent, Uzbekistan) from January 2024 to May 2025. Thirteen biological samples were obtained from patients with aortic pathologies: aneurysms combined with atherosclerotic lesions (n=5), Marfan syndrome (n=5), rupture of the sinus of Valsalva (n=2), and Takayasu syndrome (n=1). This unique cohort represents the first comparative study of these four pathologies using standardized quantitative morphometry in an emergency surgical setting. Histological preparations were examined using a ZEISS Axiostar Plus fluorescence microscope. Quantitative analysis was performed using Fiji by ImageJ software. Statistical analysis was conducted using SPSS 23.0 with the Kruskal-Wallis test and Mann-Whitney post-hoc comparisons.
Results: In Marfan syndrome, focal destruction of elastic lamellae in the tunica media, cystic medial degeneration, and uneven accumulation of glycosaminoglycans were identified. In sinus of Valsalva aneurysms, uneven thickness of collagen and reticulin fibers with pronounced edema of the middle layer were observed. Takayasu syndrome demonstrated inflammatory infiltration of the middle layer and a marked decrease in smooth muscle fibers. Patients with atherosclerosis showed severe calcification of the middle layer and presence of cholesterol crystals.
Conclusion: Morphological examination of the aortic wall in various pathologies revealed significant differences in structure and composition of wall components, confirming the heterogeneity of aortic aneurysm pathogenesis. This study establishes the first quantitative morphometric thresholds for differential diagnosis: elastic fiber density <20% indicates Marfan syndrome, inflammatory cell count >250 cells/mm² suggests Takayasu arteritis, and calcification >40% of medial area characterizes atherosclerotic aneurysms. These findings provide a foundation for developing differentiated therapeutic approaches and rapid diagnostic protocols in emergency surgical settings.
Key words: Aorta, aortic aneurysm, Marfan syndrome, Takayasu syndrome, connective tissue, medial degeneration,
pathomorphology
|
Highlights This investigation presents several unique contributions to existing knowledge: 1. The first comprehensive morphometric comparison of four distinct aortic pathologies using standardized digital analysis methods in an emergency surgery setting; 2, Novel quantitative parameters for differential diagnosis including elastic fiber density, inflammatory cell counts, and glycosaminoglycan distribution patterns; 3. Direct radiological-pathological correlation combining preoperative CT characteristics with detailed histological findings; 4. Establishment of specific threshold values for morphological parameters that could facilitate rapid intraoperative or post-surgical diagnosis in emergency scenarios where genetic testing may not be immediately available. |
![]()
Graphical abstract
Introduction
Dissection of the ascending aorta or aortic root leads to catastrophic consequences associated with high mortality (1). Aneurysmatic dilatation of the ascending aorta or aortic root is associated with an increased risk of aortic dissection, though the progression is not inevitable and depends on multiple factors including aneurysm size, growth rate, and underlying etiology (2). The aorta, now recognized as the "24th organ" due to its complex physiological functions and systemic implications, plays a crucial role in cardiovascular health (3). Aortic root aneurysms represent a particularly complex structure due to their anatomical location and are associated with higher mortality compared to ascending aortic aneurysms (4).
This increased mortality is attributed to several factors: the proximity to coronary arteries, which increases the risk of coronary compromise during dissection (5, 6); frequent involvement of the aortic valve requiring simultaneous valve replacement (7); and the technical complexity of surgical repair, which often necessitates composite valve-graft replacement with coronary artery reimplantation (8). The more extensive surgical intervention required for aortic root pathology, including the need for coronary button reimplantation and valve replacement, significantly increases operative time and perioperative complications compared to isolated ascending aortic replacement (4, 9).
The recent European Society of Cardiology (ESC) guidelines have specifically addressed the growing importance of genetic and congenital disorders in aortic pathology, providing recommendations for screening and tailored management in conditions such as Marfan and Loeys-Dietz syndromes (10). Similarly, the 2022 AHA/ACC aortic management guidelines emphasize the role of familial and genetic screening, recommending family screenings with genetic testing or aortic imaging of first-degree relatives of patients diagnosed with ascending thoracic aortic aneurysm or aortic root aneurysm or aortic dissection to identify individuals most at risk for aortic disease (11).
This heterogeneity of outcomes may be attributed to local structural differences and embryological developmental variations in the aortic wall, as well as differences in local pressure dynamics. Local damage to the ascending aorta most commonly occurs in the right lateral wall, where the greatest resistance forces and wall tension are exerted (12). In aortic root dissections, the tear often begins in the non-coronary cusp region (13). Despite structural and functional differences between ascending aortic and aortic root lesions, current treatment approaches for both pathologies remain similar (14, 15). Additionally, rare but fatal vasculitic conditions such as Hughes-Stovin syndrome, a Behcet variant that can affect the aorta and its branches, represent important differential diagnoses in inflammatory aortic pathology (16).
Study rationale
Despite significant advances in cardiovascular surgery and imaging diagnostics, the morphological basis for differential diagnosis of aortic aneurysms remains incompletely understood. Current clinical practice often relies on imaging findings and clinical presentation, but lacks standardized histopathological criteria for distinguishing between various etiological forms of aortic pathology. This knowledge gap is particularly critical in emergency surgical settings where rapid diagnosis is essential for optimal patient management, yet genetic testing results may not be immediately available. Furthermore, existing literature predominantly focuses on single pathological conditions rather than comparative morphological analysis, limiting our understanding of distinguishing features between different aortic diseases. The development of quantitative morphological markers could significantly improve diagnostic accuracy, facilitate appropriate surgical planning, and guide postoperative management strategies.
Study importance
Understanding the specific morphological characteristics of different aortic pathologies is crucial for several reasons: (1) it enables rapid differential diagnosis in emergency situations when genetic or molecular testing is not feasible; (2) it provides insight into disease-specific pathophysiology that could inform targeted therapeutic approaches; (3) it establishes objective criteria for pathologists to distinguish between similar-appearing conditions; and (4) it contributes to the development of personalized medicine approaches based on underlying tissue pathology.
Aim of the study
The aim of this study was to identify specific morphological criteria that could serve as differential diagnostic markers for various etiological forms of aortic aneurysms through comprehensive quantitative morphometric analysis and radiological-pathological correlation.
Methods
Study design and setting
This observational study was conducted at the Pathology Department of the Republican Research Center for Emergency Medicine (Tashkent, Uzbekistan) from January 2024 to May 2025. Study population Thirteen biological samples were collected from patients with various aortic pathologies who underwent emergency surgical intervention for acute aortic syndrome.
The study population included:
• Aneurysms with atherosclerotic lesions: 5 cases
• Marfan syndrome: 5 cases
• Sinus of Valsalva rupture: 2 cases
• Takayasu syndrome: 1 case
Patients` groups:
• Group 1: Systemic inflammatory and non-inflammatory pathologies (n=8): Marfan syndrome (n=5), sinus of Valsalva aneurysm rupture (n=2), and Takayasu syndrome (n=1)
• Group 2: Elderly patients with systemic atherosclerosis and malignant hypertension (n=5)
Ethical considerations
All study procedures were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (2013 revision) and complied with the legislation of the Republic of Uzbekistan and institutional guidelines. The study was approved by the local Ethics Committee of the Republican Research Center for Emergency Medicine (protocol No. 5, dated February 12, 2024). Informed consent was obtained from all patients for the use of their biological material for research purposes.
Baseline variables
We collected following baseline variables as age, sex, diagnosis, comorbidities and type of surgery.
Diagnostic criteria and patient classification
All diagnoses were established using internationally recognized criteria:
Marfan Syndrome (n=5): Diagnosis was established according to the revised Ghent nosology (2010) (17), requiring presence of aortic criterion (aortic root Z-score =2.0) combined with ectopia lentis, systemic score =7 points, or positive family history.
Additional confirmatory features included were: positive family history (n=3), ectopia lentis (n=2), and characteristic skeletal features (n=4). Genetic testing for FBN1 mutations was performed in 3 patients, with pathogenic variants identified in 2 cases.
Takayasu Arteritis (n=1): Diagnosis met the American College of Rheumatology (1990) criteria (18), including age at disease onset =40 years, arteriogram abnormality showing narrowing of aorta and primary branches, and elevated inflammatory markers (ESR >50 mm/h). Clinical presentation included claudication, diminished pulses, and arterial bruits.
Atherosclerotic Aneurysms (n=5): Diagnosis based on age >60 years with cardiovascular risk factors, absence of connective tissue disease features, characteristic imaging findings (calcified, tortuous aorta), and histological confirmation of atherosclerotic changes.
Sinus of Valsalva Aneurysms (n=2): Diagnosed by echocardiography and computed tomography (CT) angiography showing localized dilatation of aortic sinus, absence of systemic connective tissue disease, and no family history of aortic disease.
Surgical procedures
Patients underwent following surgery procedures:
•Bentall procedure (composite aortic root replacement): 6 cases (46.2%)
• Ascending aortic replacement with valve-sparing: 4 cases (30.8%)
• Patch repair of sinus of Valsalva: 2 cases (15.4%)
• Total arch replacement: 1 case (7.7%)
Imaging data collection
Preoperative computed tomography angiography (CTA) was performed in all patients using a 64-slice CT scanner. Aortic dimensions were measured at standardized anatomical landmarks: aortic root at the level of sinus of Valsalva, ascending aorta at its widest point, and aortic arch. In addition, we evaluated the calcifications, dilatation, wall thickness and presence of irregular contour of aorta. Images were analyzed by experienced radiologists blinded to histological findings.
Histological processing
Aortic tissue specimens were provided by the Department of Cardiac Surgery. To ensure optimal tissue preservation, specimens were processed according to standardized protocol: immediate placement in fixative within 15-30 minutes of excision (table-probe time), fixation duration of 12-24 hours, and processing within 48 hours of collection. Biological material was fixed in 4% neutral formalin solution in phosphate buffer (pH 7.4) at room temperature, followed by standard paraffin embedding techniques (19). Serial sections of 4-5 µm thickness were cut and mounted on glass slides.
Histological Staining
Multiple staining techniques were employed for comprehensive tissue analysis:
• Hematoxylin and eosin (H&E) for general morphology and cellular detail
• Masson's trichrome for collagen fiber visualization and differentiation
• Periodic acid-Schiff (PAS) reaction for glycosaminoglycans and basement membrane components
• Verhoeff-van Gieson stain for elastic fiber assessment
Morphological parameters
Systematic histological analysis was focused on the following specific parameters:
Structural Components:
• Elastic fiber integrity and density in the tunica media
• Collagen fiber thickness and distribution patterns
• Smooth muscle cell density and arrangement
• Reticulin fiber organization
• Aortic wall thickness measurements
• Medial edema and tissue separation
• Hyalinosis and fibrinoid degeneration
Pathological changes
•Cystic medial degeneration and mucoid accumulation
•Inflammatory cell infiltration (lymphocytes, macrophages, plasma cells)
•Calcium deposition and calcification patterns
• Cholesterol crystal formation and lipid accumulation
• Cell destruction and necrosis
• Fibrosis and collagen irregularity
Quantitative Assessment
•Wall thickness measurements at standardized locations
•Elastic fiber density (percentage of total medial area)
• Inflammatory cell count (cells per mm²)
•Calcified area as percentage of total cross-sectional area
•Glycosaminoglycan distribution coefficient of variation
Histological examination was performed using a ZEISS Axiostar Plus fluorescence microscope. All evaluations were conducted by experienced pathologists blinded to clinical diagnosis.
Statistical analysis
Quantitative analysis of histological changes was performed using Fiji by ImageJ software. Statistical data processing was conducted using SPSS 23.0. Data distribution was assessed using the Shapiro-Wilk test. Since data distribution deviated from normal, the Kruskal-Wallis test was used for comparison between groups, followed by Mann-Whitney test for pairwise comparisons.
Results are presented as median and interquartile range (Me (Q1; Q3)) for non-parametric data, or mean (standard deviation, SD) for normally distributed variables. Differences were considered statistically significant at p<0.05.
Results
The study included 13 patients with acute aortic pathology, with a mean age of 65.5 (12.3) years (range: 45-78 years). Male patients predominated (n=9, 69.2%) compared to females (n=4, 30.8%). Age was distributed by pathology as following:
• Marfan syndrome: 42.6 (8.2) years (range: 28-52 years)
• Atherosclerotic aneurysms: 72.4 (6.8) years (range: 63-82 years)
• Takayasu syndrome: 35 years
• Sinus of Valsalva aneurysms: 58.5 (12.0) years (range: 50-67 years)
The patients with atherosclerotic aneurysms were older.
Comorbidities: Hypertension was present in 8 patients (61.5%), diabetes mellitus in 5 patients (38.5%), coronary artery disease in 4 patients (30.8%), and chronic kidney disease in 2 patients (15.4%).
Surgical Procedures
All patients underwent emergency aortic surgery with the following procedures performed:
Bentall Procedure (Composite Aortic Root Replacement) - 6 cases (46.2%);
• Marfan syndrome: 4 cases;
• Atherosclerotic aneurysm: 2 cases Ascending Aortic Replacement with Valve-Sparing (David Procedure) - 4 cases (30.8%);
• Marfan syndrome: 1 case;
• Atherosclerotic aneurysm: 3 cases Patch Repair of Sinus of Valsalva - 2 cases (15.4%);
• Sinus of Valsalva aneurysm rupture: 2 cases; Total Arch Replacement - 1 case (7.7%);
• Takayasu syndrome: 1 case
Histological characteristics
As can be seen from Table 1, the most characteristic histological features by pathology are:
Atherosclerosis:
• Calcium deposition: 5/5 (100%) - most characteristic
• Atherosclerotic plaques: 5/5 (100%)
• Fibrosis: 5/5 (100%)
• Cholesterol crystals: 4/5 (80%)
|
Table 1. Histological characteristics |
||||
|
Histological Feature |
Atherosclerosis (n=5) |
Marfan Syndrome (n=5) |
Sinus of Valsalva(n=2) |
Takayasu Syndrome (n=1) |
|
Calcium deposition |
5/5 (100%)
|
1/5 (20%) |
0/2 (0%) |
0/1 (0%) |
|
Cholesterol crystals
|
4/5 (80%)
|
0/5 (0%)
|
0/2 (0%)
|
0/1 (0%)
|
|
Atherosclerotic plaques
|
5/5 (100%)
|
0/5 (0%)
|
0/2 (0%)
|
0/1 (0%) |
|
Smooth muscle destruction |
3/5 (60%)
|
5/5 (100%)
|
2/2 (100%)
|
1/1 (100%)
|
|
Elastic fiber fragmentation
|
2/5 (40%)
|
5/5 (100%)
|
1/2 (50%)
|
1/1 (100%)
|
|
Cystic medial degeneration |
1/5 (20%)
|
5/5 (100%) |
0/2 (0%) |
0/ (0%) |
|
Glycosaminoglycan accumulation |
1/5 (20%)
|
5/5 (100%)
|
2/2 (100%)
|
0/1 (0%)
|
|
Inflammatory infiltration |
2/5 (40%)
|
0/5 (0%)
|
1/2 (50%)
|
1/1 (100%)
|
|
Collagen fiber irregularity |
3/5 (60%)
|
4/5 (80%)
|
2/2 (100%)
|
1/1 (100%)
|
|
Medial edema |
1/5 (20%) |
2/5 (40%) |
2/2 (100%) |
1/1 (100%) |
|
Hyalinosis |
4/5 (80%) |
3/5 (60%) |
0/2 (0%) |
0/1 (0%) |
|
Fibrosis |
5/5 (100%) |
2/5 (40%) |
1/2 (50%) |
1/1 (100%) |
Marfan Syndrome:
• Smooth muscle destruction: 5/5 (100%) - most characteristic
• Elastic fiber fragmentation: 5/5 (100%) - most characteristic
• Cystic medial degeneration: 5/5 (100%) - most characteristic
• Glycosaminoglycan accumulation: 5/5 (100%) - most characteristic
Sinus of Valsalva Aneurysm:
• Smooth muscle destruction: 2/2 (100%) - most characteristic
• Glycosaminoglycan accumulation: 2/2 (100%) - most characteristic
• Collagen fiber irregularity: 2/2 (100%) - most characteristic
• Medial edema: 2/2 (100%) - most characteristic
Takayasu Syndrome:
• Inflammatory infiltration: 1/1 (100%) - most characteristic
• Smooth muscle destruction: 1/1 (100%)
• Elastic fiber fragmentation: 1/1 (100%)
• Fibrosis: 1/1 (100%)
Quantitative morphometric analysis
Morphometric analysis revealed significant differences between pathological groups in key
structural parameters:
• Elastic fiber content: Marfan syndrome showed marked reduction (18.2 (4.5)%) compared to
atherosclerotic group (12.8 (3.2)%, p<0.05)
• Smooth muscle cell density: Significantly decreased in Takayasu syndrome (145 (32) cells/mm²)
compared to other groups (p<0.01)
• Collagen fiber thickness: Highly variable in sinus of Valsalva aneurysms (median: 4.8 (2.9; 7.1)
µm) versus uniform distribution in controls
• Inflammatory cell infiltration: Most pronounced in Takayasu syndrome (289 (67)
cells/mm²) with minimal presence in other conditions (15-45 cells/mm²).
Radiological-pathological correlation
The correlation between preoperative CT findings and histological changes revealed distinct patterns (Table 2): Marfan syndrome showed smooth symmetric dilatation on CT corresponding to diffuse cystic medial degeneration; atherosclerotic aneurysms displayed calcified, irregular walls matching extensive calcification (45.8 (12.4)% of medial area); Takayasu syndrome demonstrated wall thickening and enhancement correlating with inflammatory infiltration (289 (67) cells/mm²).
|
Table 2. Correlation of preoperative CTA characteristic patterns with histological findings |
|||
|
Pathology |
Aortic root (mm) |
Ascending aorta (mm) |
CT characteristics |
|
Marfan syndrome
|
52.4 (6.8)
|
58.3 (7.2)
|
Smooth dilatation, thin walls
|
|
Atherosclerotic |
45.2 (4.3) |
55.1 (5.8) |
Calcifications, irregular contour |
|
Takayasu |
38.5 |
42.0 |
Wall thickening, enhancement |
|
Sinus of Valsalva
|
41.2 (3.1)
|
35.8 (2.4)
|
Localized sinus dilatation
|
|
CT- computed tomography, CTA- computed tomography angiography |
|||
Histological analysis
Marfan syndrome
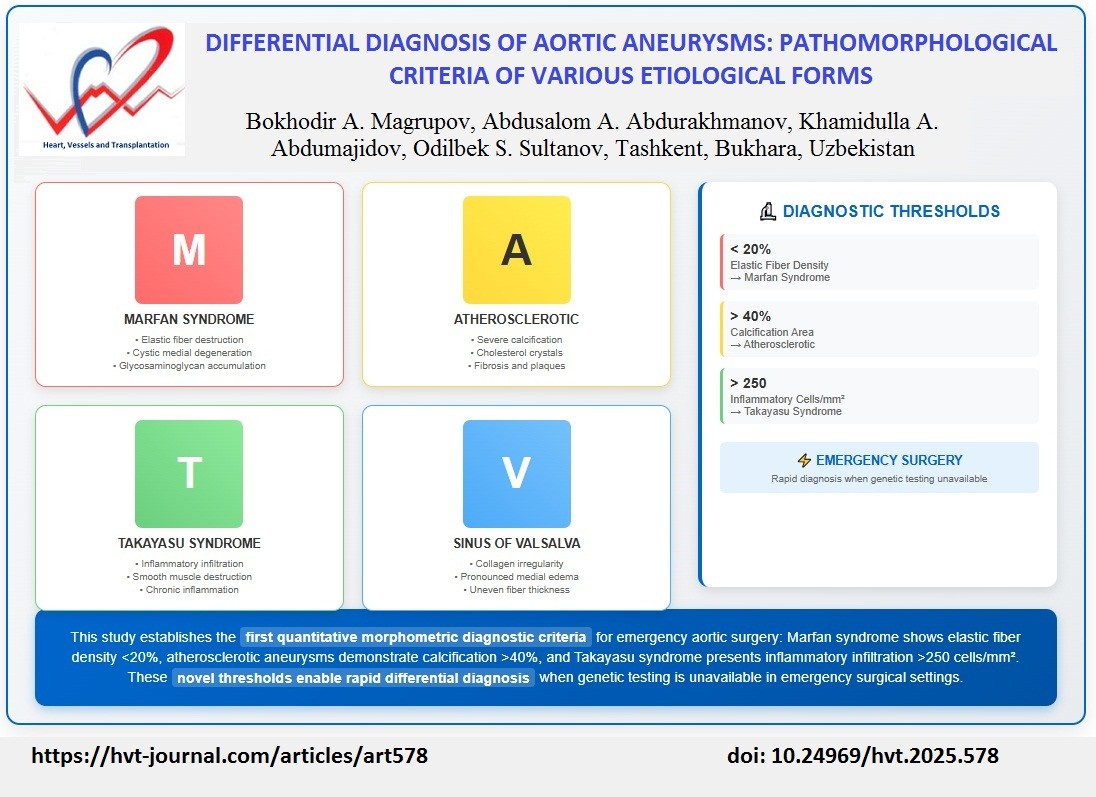
Morphological examination revealed focal destruction of elastic lamellae in the tunica media (Fig.1A), destruction of smooth muscle cells with structural disorganization presenting as cystic medial degeneration (Fig.1B), and uneven accumulation of neutral glycosaminoglycans.
Figure 1B shows the typical pattern of cystic medial degeneration of the aortic media: presence of cysts filled with PAS-positive material and pronounced disruption of medial structure, morphologically confirming deep destructive processes in the vessel wall.
Quantitative analysis demonstrated significantly reduced elastic fiber density (18.2 (4.5)%) and increased glycosaminoglycan content (35.7 (8.9)% of total medial area) compared to control specimens. Additional significant findings included high-density collagen fibers and absence of elastin fibers. Areas of hyalinosis, diffuse elastic structure destruction with basophilic ground substance, and overall reduction in smooth muscle cells in the tunica media were also observed.
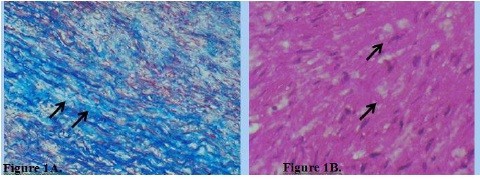
Figure 1. A. Focal destruction of elastic fibers in the aortic tunica media in Marfan syndrome. Arrows indicate: (1)
areas of elastic fiber fragmentation, (2) loss of elastic lamellae continuity, (3) intact elastic fibers for comparison.
Masson's trichrome stain. ×100. B. Cystic medial degeneration with accumulation of PAS-positive material in
Marfan syndrome. Arrows indicate: (1) cystic spaces filled with PAS-positive material, (2) disrupted medial
architecture, (3) accumulated glycosaminoglycans (magenta). PAS reaction. ×100.
Sinus of Valsalva aneurysms
Uneven thickness of collagen and reticulin fibers (Fig.2A) was noted, with uneven accumulation of neutral glycosaminoglycans and pronounced edema of the middle layer with connective tissue fiber separation. The uneven thickness of collagen and reticulin fibers represents pathological changes in the connective tissue matrix of the vascular wall, characteristic of aneurysms, chronic degenerative processes, and hereditary connective tissue diseases.
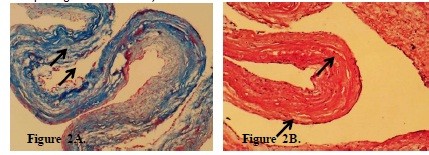
Figure 2. A. Uneven thickness of collagen fibers in sinus of Valsalva aneurysm. Arrows indicate: (1) thickened
collagen bundles, (2) areas of collagen thinning, (3) normal collagen architecture for comparison. Masson's
trichrome stain. ×100. B. Uneven distribution of glycosaminoglycans in sinus of Valsalva aneurysm. Arrows
indicate: (1) areas of intense PAS-positive staining, (2) regions with sparse glycosaminoglycan distribution, (3)
focal accumulation patterns. PAS reaction. ×100.
Figure 2B demonstrates focal, uneven distribution of neutral glycosaminoglycans in the vessel wall, detected by PAS reaction. Morphometric analysis revealed irregular glycosaminoglycan distribution patterns with coefficient of variation of 67.3 (15.2)%, significantly higher than in normal aortic tissue (p<0.001). This represents a morphological marker of degeneration and disorganization of the extracellular matrix, contributing to reduced vascular wall strength and elasticity.
Takayasu Syndrome
Inflammatory infiltration of the middle layer of varying severity (Fig. 3A), marked decrease in smooth muscle fibers (Fig. 3B), and reduction of PAS-positive material were observed.
Figure 3A demonstrates inflammatory infiltrate in the aortic media, with inflammatory cell density of 289 (67) cells/mm², indicating active inflammatory process in the vascular wall. Figure 3B shows marked reduction in smooth muscle fibers (145 (32) cells/mm² versus 380 (45) cells/mm² in controls, p<0.001), a key feature of degenerative changes characteristic of inflammatory aortic diseases, aneurysms, and hereditary connective tissue pathologies.
Atherosclerotic group
In elderly patients with atherosclerotic vascular wall lesions, distinct calcium deposits were visible in the
tunica media, appearing as intensely stained homogeneous or granular masses of pink or purple color (Fig.4A).
Figure 3. A. Inflammatory infiltration of the aortic tunica media in Takayasu syndrome. Arrows indicate: (1)
inflammatory cell infiltrates, (2) medial smooth muscle disruption, (3) areas of chronic inflammation with fibrosis.
Hematoxylin and eosin stain. ×100. B. Sharp decrease in the number of smooth muscle fibers in Takayasu
syndrome. Arrows indicate: (1) residual smooth muscle fibers (red), (2) areas of smooth muscle loss, (3)
replacement fibrotic tissue (blue). Masson's trichrome stain. ×100.
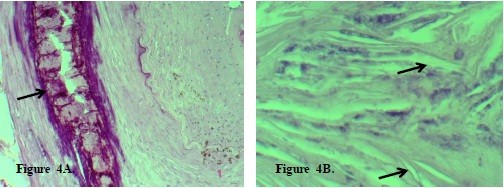
Figure 4. A. Calcification of the arterial tunica media in atherosclerosis. Arrows indicate: (1) calcium deposits (dark
purple masses), (2) calcified elastic lamellae, (3) surrounding fibrotic tissue with inflammatory infiltrate.
Hematoxylin and eosin stain. ×100. B. Cholesterol crystals in the arterial wall in atherosclerosis. Arrows indicate:
(1) needle-shaped cholesterol crystals, (2) lipid-laden foam cells, (3) necrotic atherosclerotic plaque core.
Hematoxylin and eosin stain. ×400.
Calcification of the tunica media (Fig. 4A) is a characteristic feature of late-stage atherosclerosis.
Quantitative analysis revealed calcified area comprising 45.8 (12.4)% of the total medial cross-sectional area. Calcium deposition in the arterial wall leads to loss of elasticity, increases vessel rigidity, and contributes to complications such as impaired blood flow, thrombosis, or arterial rupture.
Figure 4B shows cholesterol crystals, with crystal density of 180 (35) crystals/mm², a characteristic feature of atherosclerotic plaques, indicating pronounced lipid metabolism disorders, tissue destruction, and atherosclerotic process progression.
Discussion
Novel contributions
This study represents the first comprehensive quantitative morphometric comparison of four distinct aortic pathologies in an emergency surgical setting, establishing specific diagnostic thresholds that could facilitate rapid pathological diagnosis when genetic testing is unavailable or time-sensitive decisions are required. Our findings reveal distinct morphological changes in the aortic wall across different pathologies, consistent with previous research but providing novel quantitative parameters. The observed reduction in elastin fiber content and increased collagen structure density in ascending aortic aneurysms aligns with studies by Cattell et al. (20) and Tsamis et al. (21), who noted that aneurysmatic changes involve extracellular matrix disorganization and remodeling, accompanied by reduced vascular wall elasticity and strength. However, our study uniquely establishes that elastic fiber density below 20% specifically indicates Marfan syndrome, providing a quantitative diagnostic criterion not previously described.
In Marfan syndrome cases, we identified focal elastic destruction, cystic medial degeneration, and reduced smooth muscle cell count, corresponding to descriptions by Amalinei et al. (22) and Nataatmadja et al. (23), who linked these changes to impaired synthesis and degradation of extracellular matrix components. Our findings align with recent comprehensive analysis demonstrating similar patterns of cystic medial degeneration and elastic fiber disruption (24).
The correlation between CT imaging characteristics (smooth aortic dilatation with preserved wall architecture) and our histological findings (focal elastic destruction with 18.2 (4.5)% elastic fiber content) supports the concept of progressive connective tissue degradation in Marfan syndrome. This radiological-pathological correlation validates the use of CT imaging for monitoring disease progression and surgical planning, representing the first study to establish specific quantitative CT-histology correlations in emergency aortic surgery patients.
Similar morphological findings in patients with bicuspid aortic valve (25, 26) indicate common pathogenetic mechanisms in aneurysm formation across hereditary and acquired pathologies.
In atherosclerotic aortic changes, we identified pronounced calcification and fibrosis areas, confirmed by Carmo et al. (27) and Nesi et al. (28), who noted that calcification and sclerosis are morphological markers of late-stage atherosclerotic processes. Our study advances this knowledge by establishing that calcification exceeding 40% of medial cross-sectional area specifically characterizes atherosclerotic aneurysms, providing a novel quantitative diagnostic parameter. Inflammatory infiltration in Takayasu syndrome, as described by Goudot et al. (29), emphasizes inflammation's role in vascular wall remodeling. We uniquely demonstrate that inflammatory cell density above 250 cells/mm² serves as a reliable marker for Takayasu arteritis, offering the first quantitative threshold for this rare condition in emergency surgical specimens.
Methodological innovation
Quantitative analysis using digital methods (Fiji by ImageJ) provided objective assessment of change severity, consistent with current morphometric research trends (30, 31). However, our standardized protocol for emergency surgical specimens and correlation with preoperative imaging represents a novel approach that could be implemented in clinical practice for rapid diagnosis.
Clinical implications
The establishment of specific quantitative thresholds enables rapid pathological assessment in emergency scenarios, potentially influencing surgical approach and postoperative management. This represents the first study to provide actionable quantitative criteria for differential diagnosis of aortic pathologies in emergency surgical settings.
Study limitations
We have to acknowledge the small sample size of the study. Further studies are needed with larger sample size and validation of quantitative criteria of differential diagnosis of aortic pathology in clinical setting.
Conclusion
Morphological examination of the aortic wall in various pathologies revealed significant differences in structure and composition of wall components depending on the underlying disease. This study establishes the first quantitative morphometric diagnostic criteria for emergency surgical specimens: Marfan syndrome predominantly shows focal elastic destruction of the tunica media with elastic fiber density <20%, cystic medial degeneration with glycosaminoglycan accumulation, and overall smooth muscle cell reduction; atherosclerotic aneurysms are characterized by tunica media calcification >40% of cross-sectional area and uneven collagen and reticulin fiber thickness; Takayasu syndrome demonstrates pronounced inflammatory infiltration >250 cells/mm². These novel quantitative thresholds represent the first standardized morphometric criteria for rapid differential diagnosis in emergency aortic surgery settings. The morphological differences confirm the heterogeneity of aortic aneurysm pathogenesis and provide a foundation for developing differentiated treatment approaches and implementation of rapid diagnostic protocols when genetic testing is unavailable or time-sensitive surgical decisions are required.
Ethics: All study procedures were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki (2013 revision) and complied with the legislation of the Republic of Uzbekistan and institutional guidelines. The study was approved by the local Ethics Committee of the Republican Research Center for Emergency Medicine (protocol No. 5, dated February 12, 2024). Informed consent was obtained from all patients for the use of their biological material for research purposes.
Peer-review: External and internal
Conflict of interest: None to declare
Authorship: Concept and design: B.A.M., A.A.A. Data collection and processing: O.S. S. Manuscript writing: B.A.M., O.S.S.; Editing: A.A. A, H.A. A. Critical revision and approval of the manuscript for publications: all authors. Thus, all authors fulfilled all authorship criteria for publication.
Acknowledgement: The authors acknowledge and thank the Department of Cardiac Surgery for providing tissue specimens and the technical staff for their assistance in histological processing.
Funding: None to declare
Statement on A.I.-assisted technologies use- Authors declare that they did not use AI-assisted technologies in preparation of this manuscript
Availability of data and material: Contact authors, rules of fair use with acknowledgement of source or other collaboration are required.
References
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Copyright

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
AUTHOR'S CORNER

